FIGURE 5.
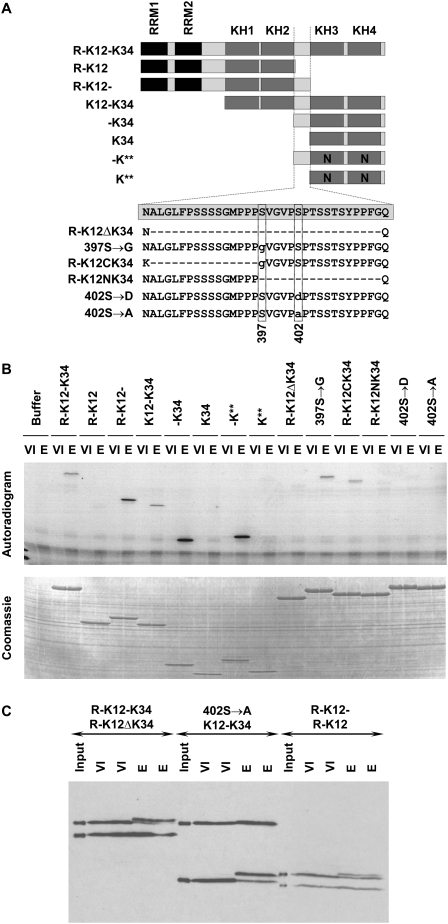
Vg1RBP is phosphorylated on Serine 402. (A) Schematic representation of recombinant Vg1RBP variants and their nomenclature. Black: RRMs, dark gray: KH domains, light gray: unstructured sequences, N: point mutations in KH domains which abrogate RNA binding. The wild-type sequence of the inter-KH linker (spanning residues 381–414) is boxed; internal deletions are showed as hyphens, point mutations are in lowercase, the position of serines 397 and 402 is indicated below. The drawing is not to scale. (B) Serine 402 is phosphorylated in vitro; 15 pmol of each of the indicated Vg1RBP variants was used as a substrate in an in vitro kinase assay using extracts derived from either stage VI oocytes (VI) or progesterone-matured eggs (E). Samples were visualized using autoradiography (top panel) and Coomassie Blue staining (bottom panel). (C) Serine 402 is phosphorylated in vivo. Pairs of equimolar recombinant Vg1RBP variants were injected into stage VI oocytes, which were then incubated overnight in the presence (E) or absence (VI) of progesterone. Protein extracts from duplicate samples were subjected to Western blot analysis using anti-His antibody alongside samples of the injected mixtures (input). Injected proteins are indicated with the larger protein above the smaller protein. Note that due to differential stability of the injected proteins, samples from R-K12/R-K12- injected cells represent two rather than one cell equivalents.