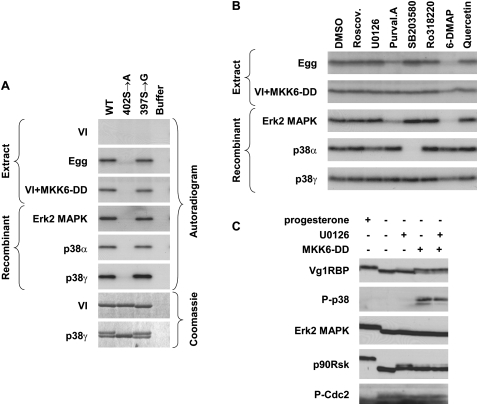
FIGURE 6.
Vg1RBP is phosphorylated by MAPK. (A) Serine 402 can be phosphorylated by both Erk2 and p38 MAP kinases; 15 pmol of recombinant full-length wild-type (WT), S402 → A or S397 → G Vg1RBP variants or buffer alone were used as substrates for in vitro kinase assays with 10 ng recombinant in vitro-activated Erk2, p38α, or p38γ or extracts made from stage VI oocytes (VI), progesterone-matured eggs (E), and stage VI oocytes injected with MKK6-DD (VI + MKK6-DD). Samples were visualized using autoradiography and Coomassie Blue staining. (B) The phosphorylation of Vg1RBP by egg extract is sensitive to Erk2 MAPK but not p38/MAPK inhibitors; 15 pmol wild-type recombinant Vg1RBP was used as a substrate for in vitro kinase assays using kinases and extracts as above in the presence of the following inhibitors: 10 μM Roscovitine, 20 μM U0126, 10 μM Purvalanol A, 10 μM SB203580, 1 μM Ro328220, 1 mM 6-DMAP, or 20 μM Quercetin. A similar volume of DMSO was used as a control. (C) In vivo activation of p38 MAPK leads to Vg1RBP phosphorylation in the absence of cell cycle progression. Stage VI oocytes were preincubated for 4 h with 50 μM U0126 or equivalent concentrations of DMSO, microinjected with 50 nL of 1.5 mg/mL MKK6-DD and incubated overnight alongside with progesterone-treated oocytes. Cells were then subjected to Western blot analysis using antisera directed against the proteins indicated on the left.