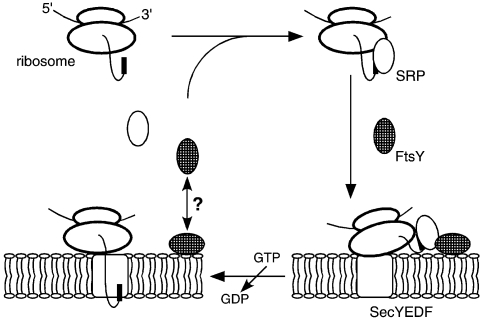
Figure 1.
The hypothetical signal recognition particle (SRP) cycle of Archaea. Proteins destined to cross the archaeal plasma membrane are synthesized with a cleavable N-terminal extension (the signal sequence, shown as a bold black line), although exceptions to this rule exist. The signal sequence is recognized by the SRP54 subunit of the SRP. Although the diagram depicts the interaction of SRP54 and the signal sequence occurring soon after the onset of protein synthesis, it remains unclear at which step this interaction takes place. The SRP–polypeptide complex is then delivered to the membrane, presumably through the affinity of the SRP for FtsY (hatched), the archaeal SRP receptor. It is presently unknown whether a soluble form of FtsY binds the complex and only then interacts with the membrane or whether a membrane-associated form of FtsY is employed. The nature of the FtsY–membrane interaction also remains to be determined. Although the role played by GTP in the archaeal SRP cycle is currently undefined, it is likely that GTP hydrolysis events mediated by SRP54 or FtsY lead to the release of SRP and FtsY from the complex and the transfer of the ribosome to the membrane-embedded SecYEDF translocation machinery, possibly with the bulk of the nascent polypeptide chain still being translated. The Sec complex, presumably responsible for both protein translocation and insertion in Archaea, may contain additional Archaea-specific subunits.