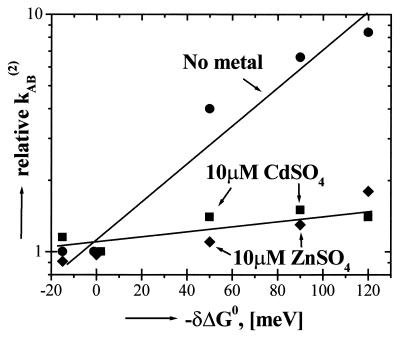
Figure 3.
The rate constant of proton-coupled second electron transfer, kAB(2), in RCs as a function of the change in redox free energy (driving force) for electron transfer in the absence and in the presence of 10 μM Zn2+ and Cd2+. Note that kAB(2) in the absence of added metals show a “Marcus”-like dependence on the electron driving force characteristic of a rate-limited electron transfer reaction as has been reported (6), whereas in the presence of Zn2+ or Cd2+, kAB(2) is approximately independent of the electron driving force, showing that proton transfer (Eq. 3) has become rate limiting. Quinones substituted into the QA site were from left to right: MQ0, menadione (2-methyl-1,4-naphthoquinone); Q10, coenzyme Q10; MQ4, menatetrenone (2-methyl-3-tetraisoprenyl-1,4-naphthoquinone); ADMNQ, 2,6-dimethyl-3-undecyl-1,4,-naphthoquinone; and ATMNQ, 2,6,7-trimethyl-3-undecyl-1,4,-naphthoquinone. Conditions were the same as in Fig. 1.