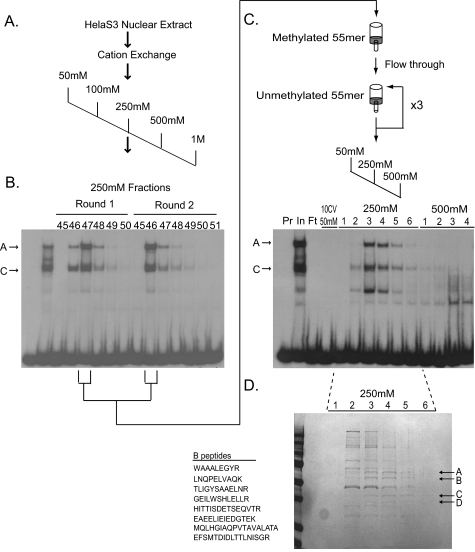
FIGURE 3.
Purification of the HS2–55bp binding activity. A, the HS2–55bp binding activity was isolated from HeLa S3 cell nuclear extract by cation exchange chromatography and a two-step DNA affinity chromatography scheme. HeLa S3 nuclear extract (415 mg) was divided in half, and each half was independently bound to a SP-Sepharose cation exchange column in 50 mm NaCl binding buffer and step eluted in 50 mm, 100 mm, 250 mm, 500 mm, and 1 m NaCl. Methylation-sensitive binding activity in the fractions was monitored by electrophoretic mobility shift assay (EMSA) using the HS-55bp probe in the presence of 100-fold molar excess of unlabeled HS2–55bp methylated at both CpG sites as a competitor. B, methylation-sensitive binding activity from each round of purification eluted with the 250 mm fractions. Representative EMSA analysis is shown. Methylation-sensitive complexes A and C are indicated. C, fractions containing peak activity from the cation exchange column were combined and negatively selected on a DNA affinity column containing the oligomerized, methylated HS2–55bp oligonucleotide. Flow-through from the methylated column was then bound to a DNA affinity column containing the oligomerized, unmethylated, HS2–55bp oligonucleotide, and eluted in 500 mm NaCl. Binding and elution from the unmethylated column were repeated twice, with a final step elution in NaCl. Fractions were analyzed by EMSA using the HS-55bp probe in the presence of 100-fold molar excess of unlabeled HS2–55bp methylated at both CpG sites as competitor. A representative analysis of the third round of purification is shown. Lanes 1, probe alone (Pr); lane 2, input (In); lane 3, flow-through (Ft); lane 4, 10 column volumes of 50 mm NaCl wash, 250 mm NaCl, and 500 mm NaCl eluted fractions. D, fractions 1–6 (250 mm NaCl) from the third round of affinity purification (see C) were trichloroacetic acid precipitated, separated on an SDS-10% polyacrylamide gel, and stained with Gel Code Blue (Pierce). Bands A–D (arrows) co-eluting with HS2 binding activity were excised and subject to MALDI MS/MS.