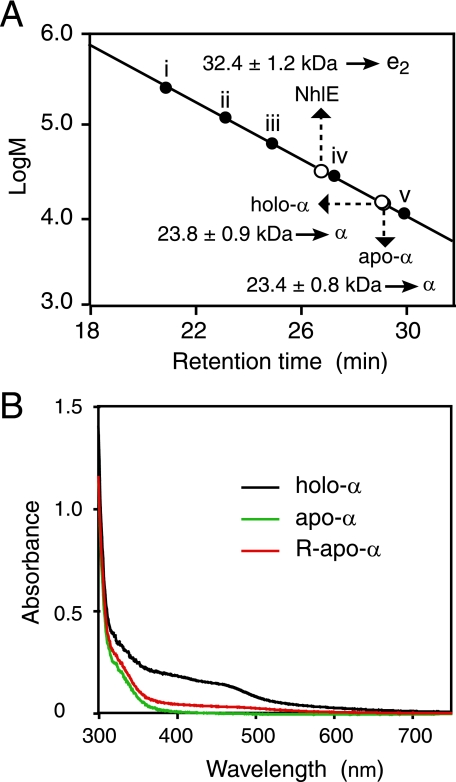
FIGURE 6.
Characterization of purified holo-α, apo-α, NhlE, and R-apo-α. A, molecular mass and structure determination of the α-subunits and NhlE derived from apo-αe2 and holo-αe2 by gel filtration on a Superose 12 HR 10/30 column. Each of nhlA and nhlE (DDBJ accession numbers X64360 (nhlA) and D83695 (nhlE)) would encode proteins of 207 amino acids (22.8 kDa) and 148 amino acids (16.9 kDa), respectively. Marker proteins: i, glutamate dehydrogenase (yeast) (290 kDa); ii, lactate dehydrogenase (pig heart) (142 kDa); iii, enolase (yeast) (67 kDa); iv, myokinase (yeast) (32 kDa); v, cytochrome c (horse heart) (12.4 kDa). Values represent the means ± S.D. for at least triplicate independent experiments. B, UV-visible spectra of the purified holo-α, apo-α, and R-apo-α.