FIGURE 9.
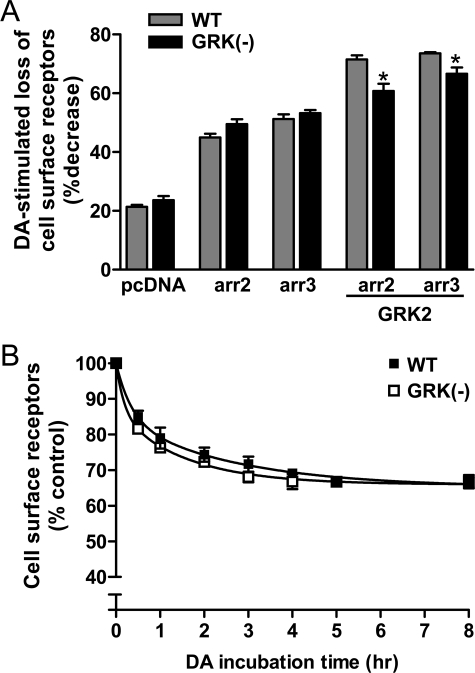
Effect of eliminating GRK phosphorylation sites on DA-induced D2 DAR internalization. A, HEK293T cells were transfected with either WT or GRK(-) mutant D2L DAR along with empty vector (pcDNA), arrestin 2 (arr2), arrestin 3 (arr3), or in combination with GRK2. The cells were incubated in the absence or presence of 10 μm DA for 1 h and then subjected to intact cell [3H]sulpiride-binding assays as described under “Experimental Procedures.” The internalization of the D2 receptor was assessed by measuring the decrease in cell surface [3H]sulpiride-binding sites, and the data are expressed as the percent loss of cell surface receptors. The values shown represent the means ± S.E. of at least three independent experiments. *, p < 0.05 compared with WT in the presence of arrestin and GRK2 overexpression. B, HEK293T cells were transfected with either WT or GRK(-) mutant D2L DARs. Cells were incubated in the absence or presence of 10 μm DA for indicated times and then subjected to intact cell [3H]sulpiride binding assays. The amount of cell surface receptor is expressed as the percentage of [3H]sulpiride binding observed in the control (no DA treatment) group. Data are represented as the means ± S.E. from at least three experiments (0–3 h). Data for 4, 5, and 8 h are from two independent experiments.