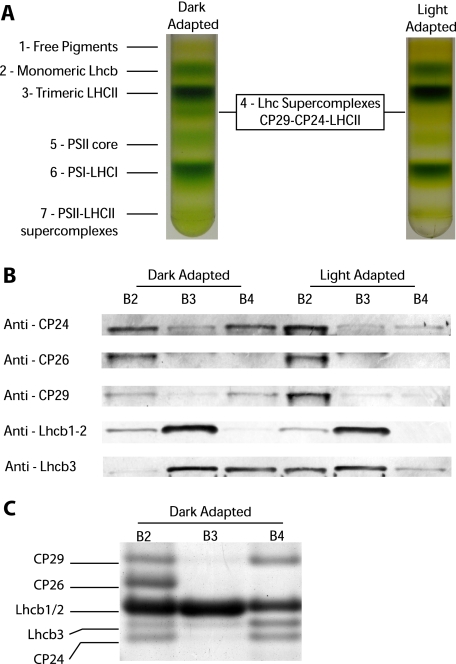
FIGURE 1.
Light-dependent dissociation of B4C protein complex in A. thaliana. A, sucrose gradient fractionations of mildly solubilized thylakoids membranes purified from dark- and light-adapted (30 min at 1500 μE) leaves. In the dark thylakoid pigment-binding complexes separate into seven distinct bands, the fourth one (B4C) being depleted in light-treated sample. B, distribution of monomeric antenna proteins in sucrose gradients between bands 2, 3, and 4 from dark- and light-adapted samples. Western blotting analysis was carried out using specific antibodies against CP24, CP26, CP29, Lhcb1-2, and Lhcb3, respectively. Samples from different bands were loaded in amounts proportional to their abundance in the sucrose gradient. In Lhcb1-2 blotting, each band was loaded with five times less protein to avoid antibody signal saturation. C, Coomassie-stained SDS-PAGE loading of equal Chl amounts (2 μg) from sucrose gradient bands 2, 3, and 4 from dark-adapted samples. Bands corresponding to CP29, CP26, Lhcb1/2, Lhcb3, and CP24, as identified by Western blotting, are indicated. Only the gel region where antenna polypeptides are migrating is shown.