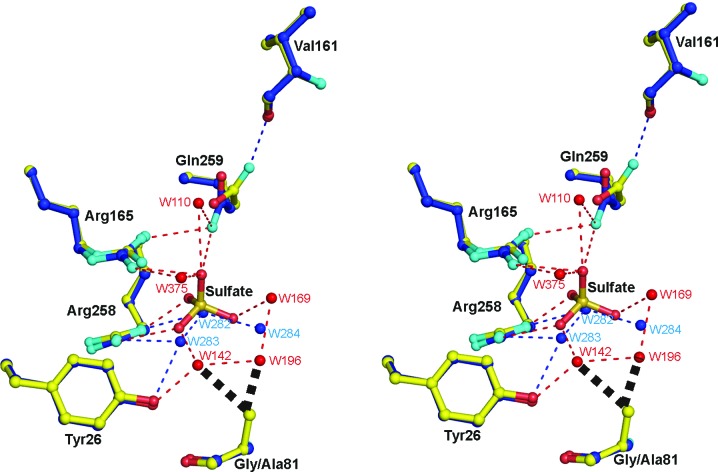
Figure 6.
Superposition of the active-site structures of the G81A mutant form of MDH-GOX2 (blue C atoms) and the mandelate-reduced MDH-GOX2 (yellow C atoms), including a bound sulfate anion in the latter, with their respective water molecules in blue and red. O and N atoms are shown in red and cyan, respectively, for both structures. Hydrogen bonds are shown as narrow dashed lines in blue for reduced MDH-GOX2 and in red for the G81A mutant proteins. The various residues are labeled in black. Side-chain atoms only are shown for each of the residues, except for Val161 and Gly/Ala81 for which main-chain atoms are also shown; the flavin and residues Tyr131 and His255 are omitted for clarity. The bold black dashed lines near the methyl group of Ala81 of G81A indicate steric repulsion of two water mocules of reduced MDH-GOX2 that are displaced by the methyl group in G81A. This diagram was prepared using PyMOL (DeLano, 2002 ▶).