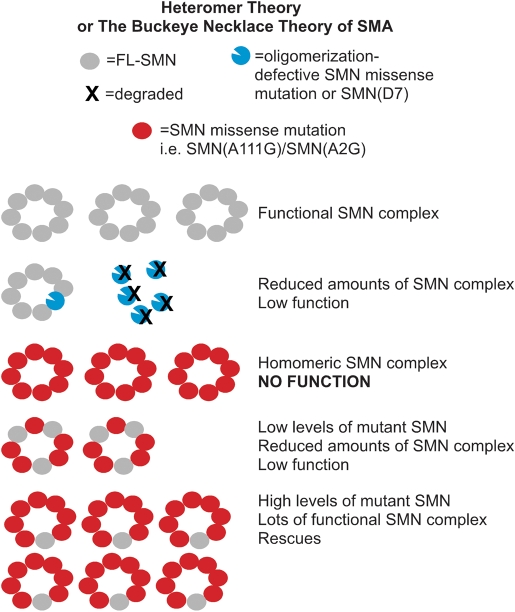
Figure 7.
The heteromer theory of SMA to explain why retention of SMN2 is critical for phenotypic rescue by mild missense alleles. Mutations of SMN defective in oligomerization or SMN(D7) are rapidly degraded and result in a severe phenotype as the level of competent SMN complexes is insufficient for motor neurons (Row 2). Mild missense mutations of SMN are those that can complement full-length SMN from SMN2. Expression of mild mutant SMN transgenes in the absence of full-length SMN results in lethality due to the inability of the mutant to function on its own (Row 3). The addition of SMN2 results in functional SMN complexes wherein full-length SMN provides a missing property necessary for the activity of the complex. Thus, any SMN complex that contains a molecule of full-length SMN will be functional and any complex that does not contain full-length SMN will not be functional. In other words, there is no dominant negative effect of SMN(A111G) and the SMN from SMN2 is limiting. The maximum number of functional SMN complexes will be obtained when SMN(A111G) levels are high and each complex contains one full-length SMN (Row 4), while lower levels of SMN(A111G) will result in complexes containing more than one full-length SMN, thus limiting the number of functional complexes (Row 5). The SMN ring is shown as an octamer based on (74) and our data suggest that the complex is more than a dimer because much greater amounts of SMN(A111G) than SMN from SMN2 are necessary to obtain full rescue (in other words the ratio is not 1:1).