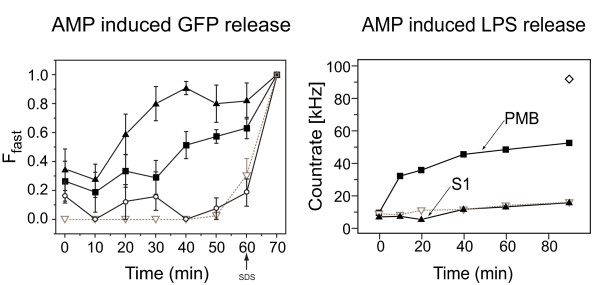
Figure 3.
Determination of green fluorescent protein release from Escherichia coli using fluorescence correlation spectroscopy. (A) Bacterial lysis assay with 500 nM S1 (solid upward triangle), 500 nM polymixin B (PMB) (solid square), no additive (open downward triangle) and negS1 (open circles) using fluorescence correlation spectroscopy (FCS). The peptide-induced leakage of green fluorescent protein (GFP) from the cytosol was observed by recording the fluorescence correlation curves and fitting it after a two-particle diffusion model. Ffast is the fraction of fast diffusing particles, that is, GFP free in solution. The fraction of slow diffusing particles, that is, GFP enclosed within bacterial cells, is given by 1-Ffast. Note that these fractions are not synonymous with mole fractions, as explained in the text. The last time point for each graph represents sodium dodecyl sulfate (SDS)-induced leakage. In contrast to a sample with no additive, S1 and PMB induced leakage of E. coli. The control peptide negS1 induced a minor amount of leakage probably due to its high hydrophobic nature. (B) Antimicrobial peptide-induced lipopolysaccharide (LPS) release. The supernatant of fluorescent-labeled LPS molecules on bacteria was analyzed in an FCS setup. The y-axis represents the fluorescence photon count rate measured in kHz, that is, thousand photon counts per second. The count rate is a measure of the amount of LPS released from bacteria. In contrast to PMB (solid square), S1 (solid upward triangle) did not induce release of LPS during lysis of E. coli. As a positive control SDS (open diamond) was added, which disrupted the membrane therefore releasing LPS. A negative control without detergent or AMP (open downward triangle) did not cause release of LPS.