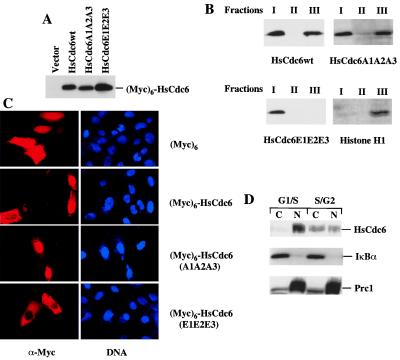
Figure 3.
Chromatin/nuclear matrix association and subcellular localization of (Myc)6-HsCdc6wt, (Myc)6-HsCdc6A1A2A3, and (Myc)6-HsCdc6E1E2E3. (A) U2OS cells were transiently transfected with DNA constructs expressing (Myc)6-HsCdc6wt, (Myc)6-HsCdc6A1A2A3, (Myc)6-HsCdc6E1E2E3, and control, (Myc)6. Lysates from indicated transfected cells were subjected to SDS/PAGE and then analyzed by immunoblotting with the 9E10 mAb. (B) Cells expressing indicated proteins in A were subjected to chromatin/nuclear matrix fractionation. The soluble fraction from the first extraction (I), the soluble fraction from the third extraction (II), and the chromatin/nuclear matrix fraction (III) from the indicated cells were subjected to SDS/PAGE and then analyzed by immunoblotting with either the 9E10 mAb or anti-histone H1 mAb. (C) U2OS cells were grown on glass coverslips and transiently transfected with DNA constructs expressing indicated proteins as in A. After fixation, samples were incubated with the 9E10 mAb. Immunofluorescence staining was performed with Texas red-conjugated goat anti-mouse antibodies, and nuclei were visualized with Hoechst dye 33258. Fluorescent images were obtained by using a fluorescence microscope. (D) Subcellular localization of endogenous HsCdc6 during the cell cycle. HeLa cells were synchronized by a “double thymidine” block in G1/S phase and then released into S/G2 phase in fresh medium for a period of 6 h. Subcellular fractionation was performed as described (42, 43). Equal amounts of cytoplasmic and nuclear proteins extracted from G1/S and S/G2 cells were subjected to SDS/PAGE and then analyzed by immunoblotting with anti-HsCdc6 antibodies (Top), anti-IκBα (a cytoplasmic protein) antibodies [Middle (42)], or anti-Prc1 (a nuclear protein) antibodies [Bottom (29)], respectively.