Abstract
Background
The widespread occurrence of feminized male fish downstream of some wastewater treatment works has led to substantial interest from ecologists and public health professionals. This concern stems from the view that the effects observed have a parallel in humans, and that both phenomena are caused by exposure to mixtures of contaminants that interfere with reproductive development. The evidence for a “wildlife–human connection” is, however, weak: Testicular dysgenesis syndrome, seen in human males, is most easily reproduced in rodent models by exposure to mixtures of antiandrogenic chemicals. In contrast, the accepted explanation for feminization of wild male fish is that it results mainly from exposure to steroidal estrogens originating primarily from human excretion.
Objectives
We sought to further explore the hypothesis that endocrine disruption in fish is multicausal, resulting from exposure to mixtures of chemicals with both estrogenic and antiandrogenic properties.
Methods
We used hierarchical generalized linear and generalized additive statistical modeling to explore the associations between modeled concentrations and activities of estrogenic and antiandrogenic chemicals in 30 U.K. rivers and feminized responses seen in wild fish living in these rivers.
Results
In addition to the estrogenic substances, antiandrogenic activity was prevalent in almost all treated sewage effluents tested. Further, the results of the modeling demonstrated that feminizing effects in wild fish could be best modeled as a function of their predicted exposure to both antiandrogens and estrogens or to antiandrogens alone.
Conclusion
The results provide a strong argument for a multicausal etiology of widespread feminization of wild fish in U.K. rivers involving contributions from both steroidal estrogens and xenoestrogens and from other (as yet unknown) contaminants with antiandrogenic properties. These results may add further credence to the hypothesis that endocrine-disrupting effects seen in wild fish and in humans are caused by similar combinations of endocrine-disrupting chemical cocktails.
Keywords: antiandrogen, endocrine disruption, estrogen, feminization, fish, testicular dysgenesis
Wildlife populations associated with the aquatic environment can be exposed to concentrations of endocrine-disrupting pollutants that are high enough to compromise their reproductive capacity (reviewed by Vos et al. 2000); this exposure may, in turn, have population-level consequences (Kidd et al. 2007). The widespread nature of these abnormalities has led to substantial interest from scientists and the general public. This concern stems, in part, from the hypothesis that reproductive diseases seen in humans are also caused by exposure to the same chemical contaminants (Skakkebaek et al. 2001). However, the actual evidence to support the wildlife–human connection is weak. Moreover, in most cases there is little evidence to link cause and effect in even a single species, let alone multiple species. Some of the best evidence has been found in riverine fish populations where feminization of wild male fish (e.g., Jobling et al. 1998) is thought to be caused predominantly by exposure to steroidal estrogens in wastewater treatment work (WWTW) effluents originating from human and animal excretion (Desbrow et al. 1998; Routledge et al. 1998), with minor contributions from other estrogenic chemicals found in WWTWs effluents, such as bisphenols and phthalates, nonylphenols (NPs) and their ethoxylates, and carboxylates (Gibson et al. 2005; Harries et al. 1997; Vajda et al. 2008; Vethaak et al. 2005).
Supporting the role of these steroidal estrogens in the feminization of wild fish, recently, a very strong correlation was shown between the predicted steroidal estrogen content of U.K. rivers and feminization in wild fish (Jobling et al. 2006). Reproductive disorders also seen in human males are, however, best induced by exposing laboratory rodents to environmentally relevant concentrations of antiandrogens and estrogens rather than to estrogens alone (Sharpe and Skakkebaek 2008; Skakkebaek et al. 2001), thus suggesting that the etiology of endocrine-disruptor–induced reproductive diseases likely differ in humans and fish. Notwithstanding this, the fact that there are > 100,000 substances in wastewater effluents (not including the different isomers of chemicals or their products of degradation), many of which have endocrine-disrupting properties other than estrogenic, makes it highly likely that the feminizing responses seen in male fish also have a multicausal etiology involving chemicals with nonestrogenic mechanisms of action. The objective of the present study, therefore, was to further explore this possibility by challenging the hypothesis that steroidal estrogens are solely responsible for widespread sexual disruption seen in wild fish in U.K. rivers. We used data on hormonal (estrogenic, antiestrogenic, androgenic, and antiandrogenic) activities and concentrations of known endocrine disruptors in WWTW effluents, together with hydrologic data, to predict hormone and antihormone concentrations in receiving waters over a wide geographic range. We then explored their relationships with sexual disruption in the wild fish living in these waters using statistical modeling. The results suggest that antiandrogenic chemicals of unknown identities are widespread in U.K. effluents and receiving waters and that, in addition to the steroidal estrogens, these constituents of WWTW effluents are likely to play a major role in causing endocrine disruption in wild fish.
Methods
Data sources
Effluent hormonal activity and chemistry
The Environment Agency’s survey of hormonal activity in 51 effluents (Environment Agency 2007) provided data on effluent chemistry from the results of a U.K. national survey of sewage treatment works effluents (locations shown in Figure 1). In that study, samples were analyzed for 17β-estradiol (E2), estrone (E1), 17α-ethinylestradiol (EE2), 4-tert-nonyl phenol (NP), and lower NP ethoxylates (NPnEO, where n = 1–5 and indicates ethoxylate chain length) and for total estrogenic, antiestrogenic, androgenic, and antiandrogenic activity in recombinant yeast screens [rYES for (anti-)estrogenic and rYAS for (anti-)androgenic activities]. The rYES and rYAS were supplied by J. Sumpter (Brunel University), and the assays were run as described by Routledge and Sumpter (1996) and Sohoni and Sumpter (1998). The detailed methods for the chemical analysis have been fully described by the Environment Agency (2007) and in other articles in which these data have also been examined (Johnson et al. 2007; Thorpe et al. 2006). Steroid estrogens were detected in all effluents at concentrations consistent with previous observations, the relative persistence of the three steroidal estrogens and differences in human excretion rates.
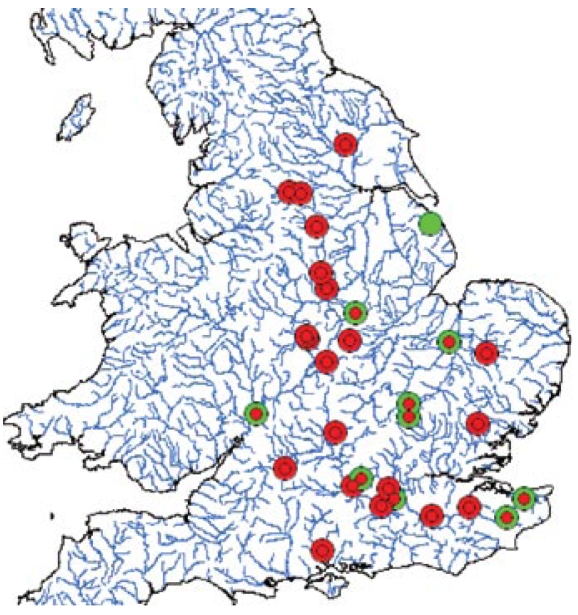
Figure 1.
Map showing the overlap in spatial distribution of estrogenic (small circles) and antiandrogenic (large circles) activity in the U.K WWTWs sampled. Red indicates the presence of activity; green indicates that no activity was found.
Estimations of (anti-)androgenic and (anti-)estrogenic activity or steroidal estrogen and alkylphenol concentrations in the river water at the fish capture sites
In the present study, we identified 30 sites where modeled predictions of exposure to steroidal estrogens, NPs, and hormonal activities in the receiving environment could be made and where fish were also captured. The sites covered a wide geographical range and had a wide variation in the proportion of the flow of the river composed of sewage effluent.
For each site, we divided the concentrations of various estrogenic chemicals and hormonal activities in the effluents of WWTWs located upstream by the dilution factor in the river at the point of fish capture to obtain estimated concentrations of each parameter in the river. Methods and supporting references have been published previously (Jobling et al. 2006).
Measurements of sexual disruption in fish
We analyzed data from the Environment Agency’s spatial survey of sexual disruption in fish (Jobling et al. 2006), which provided data on the location and prevalence of male fish with elevated plasma vitellogenin (VTG) levels, a feminized reproductive duct (fem.duct) or with developing eggs (oocytes) in the testes, and on the severity of this condition [mean relative number of oocytes in the testes (fem.index); Nolan et al. 2001] in “male” roach from each of the 30 sites (Table 1). There were 1,083 fish in total (12–71 from each location). Feminized male fish (fish with feminized ducts and/or feminized germ cells) were present at many of these sites.
Table 1.
Exposure predictions and biological impacts for 30 river sites around the United Kingdom.
| Site | E2 (ng/L) | E1 (ng/L) | EE2 (ng/L) | YES EEQ (ng/L) | Anti-YAS flutamide Eq (μg/L) | NP (μg/L) | Ovotestes (n) | Oviducts (n) | Mean
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VTG male | VTG intersex | Intersex index | |||||||||
| 1 | NQP | 0.42 | NQP | 0.14 | 9.39 | 0.15 | 0 | 1 | — | 25 | — |
| 2 | 0.3 | 5.2 | < 0.25a | 2.1 | 51.7 | 1.05 | 3 | 0 | 25 | 32 | 2.28 |
| 3 | 0.366 | 9.42 | 0.203 | 0.37 | 12.77 | 0.386 | 2 | 6 | — | 39 | 1.25 |
| 4 | < 0.066 | 5.69 | < 0.039 | 23.21 | 0 | 0.345 | 1 | 0 | 188 | NS | 1.33 |
| 5 | < 0.021 | 0.1 | < 0.012 | 1.24 | 0 | 0.09 | 5 | 0 | 496 | 2,332 | 1.54 |
| 6 | < 0.25 | < 1 | < 0.15 | 1.95 | 0 | 0.2 | |||||
| 7 | 1.308 | 3 | 0.099 | 1.63 | 6.18 | 0.318 | 4 | 3 | 310 | 305 | 1.42 |
| 8 | 0.115 | 2.13 | < 0.043 | 0.75 | 29.29 | 0.542 | 7 | 2 | 273 | 793 | 1.90 |
| 9 | NQP | 1.26 | NQP | 0.31 | 11.31 | 0.344 | 3 | 1 | 84 | 525 | 1.79 |
| 10 | NQP | 14.72 | NQP | 4.77 | 70.63 | 1.353 | 5 | 9 | 202 | 125 | 1.44 |
| 11 | 0.198 | 1.26 | 0.331 | 0.79 | 50.41 | 0.553 | 1 | 2 | 142 | 25 | 1.17 |
| 12 | < 0.08 | 5.03 | < 0.05 | 7.96 | 0 | 0.851 | 5 | 1 | 42 | 25 | 1.70 |
| 13 | < 0.005 | 0.01 | < 0.003 | 0.04 | 0 | 0.003 | 0 | 0 | 16 | — | — |
| 14 | 0.881 | 4.56 | 0.116 | 1.71 | 5.77 | 0.247 | 6 | 6 | 34 | 43 | 1.67 |
| 15 | 0.991 | 4.96 | 0.159 | 1.07 | 24.26 | 0.557 | 6 | 3 | 477 | 487 | 2.05 |
| 16 | NQP | 15.95 | NQP | 45.1 | 0 | 0.70 | 2 | 3 | 81 | 10,617 | 1.17 |
| 17 | NQP | 2.53 | NQP | 0.67 | 0 | 0.072 | 2 | 0 | 37 | 75 | 2.52 |
| 18 | NQP | 0.95 | NQP | 2.94 | 5.65 | 0.053 | 1 | 0 | 22 | 10 | 1.33 |
| 19 | 0.548 | 2.06 | 0.058 | 1.18 | 13.30 | 0.251 | 3 | 3 | 25 | 51.8 | 3.28 |
| 20 | < 0.179 | 3.1 | < 0.108 | 0.79 | 100.12 | 0.618 | 3 | 8 | 69 | 334 | 1.5 |
| 21 | < 0.152 | 15.23 | < 0.091 | 1.1 | 19.55 | 0.82 | 7 | 11 | 7,022 | 20,907 | 2.36 |
| 22 | 2.799 | 24.09 | < 0.106 | 7.09 | 72.21 | 1.303 | 7 | 1 | 41 | 186 | 3.43 |
| 23 | < 0.0013 | 0.44 | < 0.0008 | 0.85 | 0 | 0.023 | 0 | 0 | 25 | — | — |
| 24 | 1.086 | 9.84 | 0.1 | 3.94 | 75.14 | 0.796 | 4 | 6 | 422 | 272 | 2.17 |
| 25 | < 0.092 | 0.24 | < 0.0923 | 1.1 | 0 | 0.094 | 1 | 0 | 25 | 27 | 1.17 |
| 26 | < 0.052 | 3.42 | < 0.031 | 0.12 | 10.93 | 0.739 | 0 | 3 | 25 | 246 | — |
| 27 | < 0.063 | 3.56 | < 0.038 | 0.33 | 22.74 | 1.723 | 1 | 8 | 208 | 426 | 1.33 |
| 28 | 0.23 | 1.6 | 0.177 | 0.34 | 9.436 | 0.255 | 6 | 5 | 25 | 37 | 1.77 |
| 29 | NQP | 18.16 | NQP | 1.15 | 17 | 2.079 | 8 | 13 | 179 | 203 | 1.52 |
| 30 | < 0.25 | 2.0 | < 0.15 | 5.1 | 0 | 0.7 | 0 | 0 | 122 | — | — |
Abbreviations: EEQ, estradiol equivalents; flutamide Eq, flutamide equivalents; NQP, no quantifiable peak (no data); NS, not significant. Concentrations of E2, E1, EE2, and NP, as well as total estrogenic activity (EEQ) and total antiandrogenic activity (flutamide Eq) were predicted (from effluent concentrations and dilution factors).
The “less than” symbol (<) indicates effluent samples in which the concentration of the desired analyte was below the detection limit; the detection limit in each case was divided by the dilution factor of the effluent in the river at the point where the fish were captured.
Statistical methods
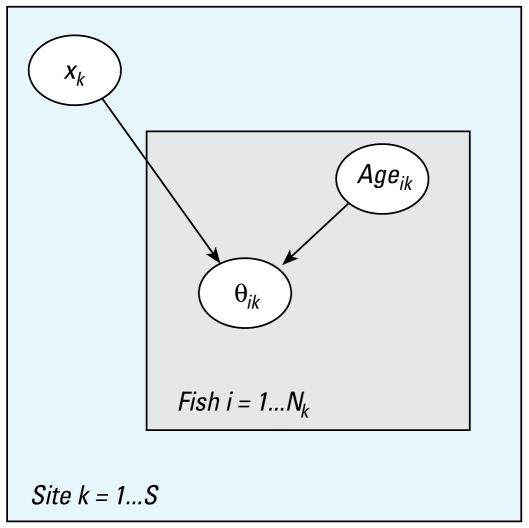
Because all of the covariates had skewed distributions, they were transformed by x → ln(x + 1), with 1 added to x to avoid difficulties with ln (0) and also so that 0 maps to 0. We used principal components analysis (PCA) to establish the patterns of variation in individual contaminants and hormonal activities in effluent samples collected. We then constructed models describing the relationship between each contaminant (alone and in combination) and each of the biological responses. These were fitted in a step-wise manner, first accounting for the effects due to estrogens and then allowing for additional effects that could be explained by antiandrogens and NP. We used logistic regression to analyze the binary response variables oocytes, fem.duct, and VTG. Generalized linear models (GLM) with gamma-distributed errors (McCullagh and Nelder 1989) fit the response fem.index well. For all responses, the data had a hierarchical structure with varying numbers (12–71) of fish sampled from the 30 sites. The concentrations of each pollutant were at site level, and the response variables were at fish level. A consequence of the data structure was that correlations between fish within sites could be anticipated and needed to be accounted for in the analysis. This was accomplished by first fitting hierarchical GLMs (Gelman and Hill 2007) with random effects for sites. For some responses, variation between sites was not significant; subsequent analyses were then simplified to ordinary nonhierarchical GLMs. An example of the general form of hierarchical model for a binary response is
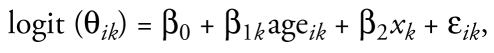 |
where θik is the probability of response for fish i in site k, and xk is the concentration of one of the pollutants at site k. This example is represented graphically in Figure 2, in which each rectangle is a level of variation.
Figure 2.
Example of the general form of hierarchical model for a binary response [logit (θik) = β0 + β1kageik + β2xk + εik, where θik is the probability of response for fish i in site k, and xk is the concentration of one of the pollutants at site k. Abbreviations: N, number of fish; S, number of sites..
Once important covariates were established using these models, we obtained smoothed estimates of the relationships using generalized additive models (GAMs) (Wood 2006). Our aim was to describe the way that covariates interacted with each other in their effect on a response. Surface plots of the fitted models indicate whether pollutants combined in an additive, synergistic, or antagonistic way in their joint effect on the response. Two covariates either a) act additively, in the sense that they affect the response independently of each other and the joint effect is the sum of their separate effects; or b) interact with each other in their effect on the response. In the latter case, the interaction can be either synergistic or antagonistic.
We performed the statistical computations using R software (R Development Core Team 2007).
Results
Exposure predictions
In vitro hormonal (rYES/rYAS) activity
We predicted that all of the river waters contained estrogenic activity and almost all also contained antiandrogenic activity (Figure 1, Table 1). Predicted estrogenic and antiandrogenic activities in the rivers ranged from 0.04 to 23.21 ng EEQ/L and from 0 to 100.12 μg flutamide equivalents/L, respectively.
Concentrations of estrogenic chemicals
After accounting for dilution, predicted steroid concentrations in the rivers receiving the effluents were between 0.01 and 24.09 ng/L for E1 and at much lower concentrations for the other two steroids. For some final effluents, we could not identify quantifiable peaks for either the steroids in the effluent extracts or the internal standards in the spiked samples, particularly EE2 (present at the lowest concentrations). These samples were noted as no quantifiable peak (NQP). For samples where the analyte was present at a concentration below detection, we assigned a value of one-half the detection limit to the effluent. After adjustment to allow for dilution in the river, these values were near zero. NP and NPnEO were also predicted to be present in river water, with concentrations of NP ranging from 0.003 to 2.079 μg/L. At only 5 of the sites, the concentration of NP was predicted to exceed 1 μg/L in river water.
Statistical analysis of the distribution of the chemicals
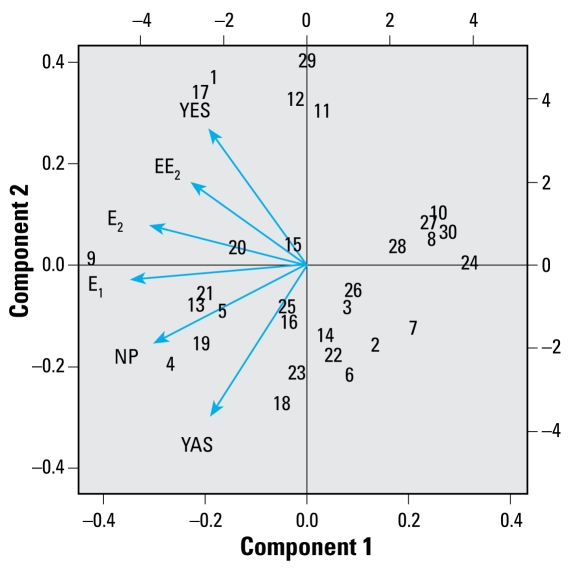
A statistical investigation of the distributions of the various pollutants and hormonal activities present at the sites sampled revealed that many of them were co-occurrent (Table 2). A consequence of the multicolinearity seen in the measurements of the various contaminants was that if the relative proportions of estrogens and antiandrogens were similar across the sites, it would have been difficult to distinguish their separate effects on fish. Fortunately, however, the results of the PCA (Figure 3) revealed that the variation in the chemical composition of the sample sites could be separated into three main components or gradients, including one component (component 2; explaining 24% of the variation in the data) that differentiated the sites with high relative proportions of estrogens from those where antiandrogens predominated. Together, the three components accounted for 87.5% of the variation in the data: Component 1 (50.3%) separated contaminated waters from background, and component 3 (12.4%) was mainly indicative of the concentration of EE2 compared with the other steroidal estrogens
Table 2.
Statistical investigation [correlation coefficients (r)] of the co-occurrence of the various pollutants and hormonal activities present in the effluents sampled.
| E1 | E2 | EE2 | NP | YAS | |
|---|---|---|---|---|---|
| E2 | 0.72# | ||||
| n = 28 | |||||
| EE2 | 0.35* | 0.56** | |||
| n = 22 | n = 22 | ||||
| NP | 0.77# | 0.45* | 0.26 NS | ||
| n = 30 | n = 28 | n = 22 | |||
| YAS | 0.48** | 0.22 NS | 0.00 NS | 0.62** | |
| n = 30 | n = 28 | n = 22 | n = 30 | ||
| YES | 0.49** | 0.44* | 0.51* | 0.15 NS | −0.25 NS |
| n = 30 | n = 28 | n = 22 | n = 30 | n = 30 |
NS, not significant. The steroidal estrogen E2 and its metabolite E1 were highly correlated (E2 is oxidized to E1). EE2 (the contraceptive pill hormone) was also associated with E2, as expected. We found no correlation between the total estrogenic (YES) and total antiandrogenic (anti-YAS) activities, indicating that the chemicals inducing these two hormonal activities are likely to be different.
p < 0.05.
p < 0.01.
p < 0.001.
Figure 3.
Plot of PCA of the chemical and (anti-)hormone composition of the sample sites showing only the first two components. The numbers on the plot are the site codes listed in Table 1. Component 1 indicates the overall level of contamination. For example site 9 is the dirtiest and site 24 the cleanest. Component 2 is high predominantly for estrogens and low predominantly for antiandrogens. The extremes on this component are sites 18 (antiandrogens) and 29 (estrogens). The arrows represent the variables; two arrows pointing in similar directions indicate that variables are correlated. The top and right axes represent standardized scores for the variables; the bottom and left axes are scores for the sites.
Statistical associations between the chemical exposure and the biological response variables
The results of the PCA analysis indicated that it may be possible to separate the modeling of the associations between the feminizing effects seen in the fish and the anti androgen exposure from those associated with estrogens. The hypothesis that antiandrogens contribute to feminization in wild fish could then be tested using statistical modeling approaches. This was done by first fitting models for each of the biological responses accounted for by estrogens and then estimating any additional effects that could be explained by antiandrogens.
Response: oocytes
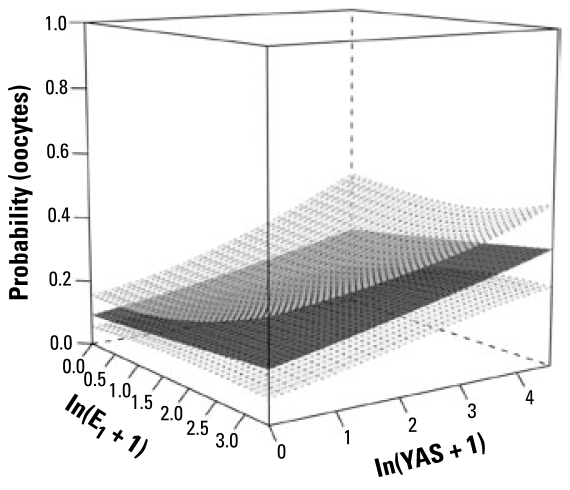
We found 94 cases of fish with oocytes in their testes. The probability of oocytes in the testis of roach was correlated positively with the age of the fish (p < 0.0001), with a sharp increase in the age-related effect when the fish were ≥ 3 years of age. Multiple logistic regressions on E1, E2, and EE2, controlling for age, revealed that E1 was the most important predictor (p = 0.004) of oocytes and that no additional significant variation in the response could be explained by E2 or EE2 (for EE2, there were only 58 cases from sites with reliable estimates of EE2 concentration). Because NP was highly correlated with E1, it accounted for no additional variation in the response either. Interestingly, we found no correlation between the total estrogenic burden [yeast estrogen screen (YES)] and the oocytes response. After allowing for E1 and age, however, there was a significant correlation between antiandrogenic activity (anti-YAS) and the oocytes response (p = 0.01). The surface plot suggested an additive effect of E1 and anti-YAS on the probability of oocytes (Figure 4). This was confirmed by the nonsignificant E1 × anti-YAS interaction term (p = 0.37) in the logistic regression model.
Figure 4.
Surface plot illustrating the results of the statistical modeling of the association between E1 and anti-YAS and the probability of oocytes in the testes of male fish. The lower and upper surfaces represent 95% confidence limits and the middle surface is the fitted mean. The suggested additive effects were confirmed by the nonsignificant E1 × anti-YAS interaction term (p = 0.37) in the logistic regression model.
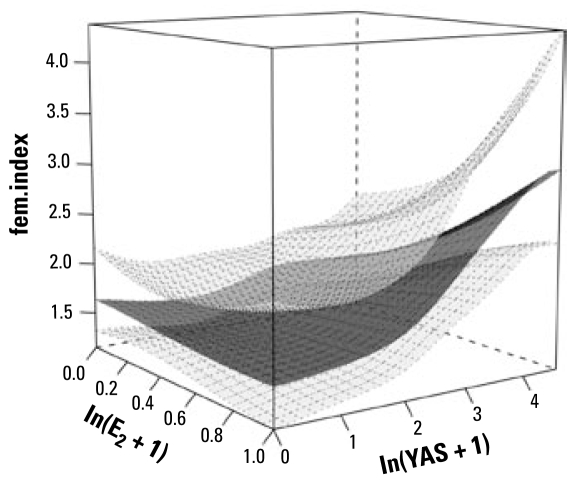
Response: fem.index
Of the 94 cases of fish with oocytes in their testes (fem.index > 0), there were only 58 cases for which there were robust measurements of EE2 in the WWTW effluents; this was insufficient for use in further statistical analysis. Disregarding EE2, multiple logistic regressions on E1 and E2 revealed that E2 was the best predictor of fem.index (p = 0.02; averaged over all values of the anti-YAS variable), and there was no effect of NP (p = 0.78) or YES (p = 0.77) on this response variable. As with the oocytes response, after allowing for the effects of E2, the additional effect of anti-YAS over E2 on the fem.index was significant (p = 0.01). The surface plot suggested a somewhat non additive effect of E2 and anti-YAS on the fem.index (Figure 5). This was confirmed by a significant negative E2 × anti-YAS interaction term (p = 0.02) in the logistic regression model.
Figure 5.
Surface plot illustrating the results of the statistical modeling of the association between exposure to E2 and anti-YAS on the feminization index in intersex fish. The lower and upper surfaces represent 95% confidence limits and the middle surface is the fitted mean. The plot indicates a somewhat nonadditive effect of E2 and anti-YAS on the fem.index. This was confirmed by a significant negative E2 × anti-YAS interaction term (p = 0.02) in the logistic regression model.
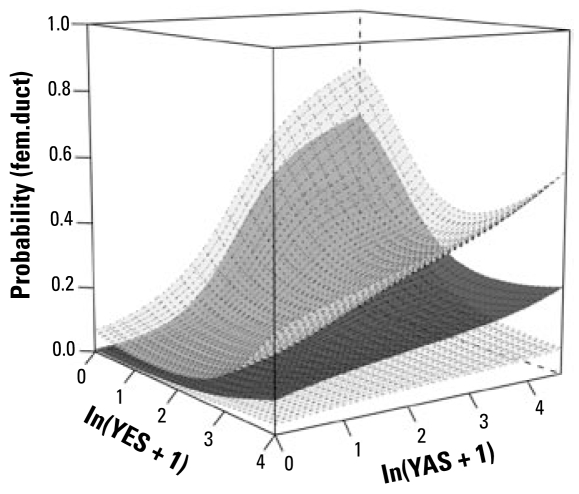
Response: fem.duct
We found significant between-site variation (p < 0.0001) for the response fem.duct. As explained in “Methods,” we accounted for this intersite variation before testing for covariate effects. Multiple logistic regressions on E1, E2, and EE2 showed that, as with the oocytes response, the overall effects of steroidal estrogens on the probability of fem.duct was best explained by E1 (p < 0.002); again, because NP was highly correlated with E1, it accounted for no additional variation in the response. The additional combined effects of both YES and anti-YAS over E1 were, however, significant (p = 0.006). The surface plot suggested an increased probability of fem.duct with increased anti-YAS, but increased YES might partially suppress this response [Figure 6; see also Supplemental Material (available online at http://www.ehponline.org/members/2009/0800197/suppl.pdf)]. This was confirmed by a significant negative YES × anti-YAS interaction term (p = 0.01) in the logistic regression model.
Figure 6.
Surface plot illustrating the results of the statistical modeling of the association between exposure to estrogenic and antiandrogenic chemicals. The lower and upper surfaces represent 95% confidence limits and the middle surface is the fitted mean. The plot indicates the additional combined effects of both YES and anti-YAS (p = 0.006) over E1 on the probability of feminization of the reproductive ducts in wild male fish. The surface plot suggested that there was an increased probability of fem.duct with increased anti-YAS, but that increased YES might partially suppress this response [see Supplemental Material, Figure 6A (available online at http://www.ehponline.org/members/2009/0800197/suppl.pdf) for two-dimensional plot]. This was confirmed by a significant negative YES x anti-YAS interaction term (p = 0.01) in the logistic regression model.
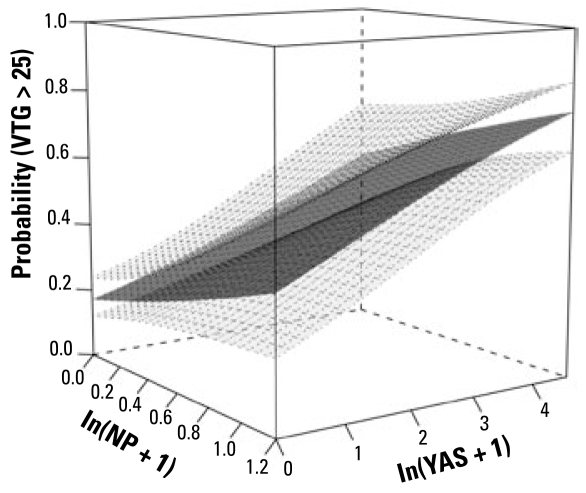
Response: VTG
We found significant between-site variation (p < 0.0001) in VTG. This was mainly because fish were sampled throughout the year and VTG varies with sampling month. After accounting for this, however, multiple logistic regressions on the steroidal estrogens E1, E2, and EE2 showed that the VTG response was best explained by E1 alone (p < 0.004). Over and above the steroidal estrogens, NP was a good predictor of the VTG response (p = 0.0002). Moreover, there was a very significant effect of anti-YAS on the VTG response (p < 0.0001). A comparison of models fitted with all possible subsets of the three variables NP, E1, and anti-YAS suggested that NP and anti-YAS were jointly the best predictors of the VTG response, although the contribution of NP was marginal (p = 0.09) over the overwhelming effect of anti-YAS on its own (p = 0.008). The surface plot suggested that, in general, the VTG response increased with increasing anti-YAS (Figure 7).
Figure 7.
Surface plot illustrating the results of the statistical modeling of the association between exposure to estrogenic and antiandrogenic chemicals on the VTG response in male and intersex fish. The lower and upper surfaces represent 95% confidence limits and the middle surface is the fitted mean. The modeling suggested that NP and anti-YAS were jointly the best predictors of the VTG response, although the contribution of NP was marginal (p = 0.09) over the overwhelming effect of anti-YAS alone (p = 0.008).
When taken together, the results of the statistical analyses suggested that male roach likely exposed to the highest concentrations of antiandrogens and/or steroidal estrogens exhibited the highest prevalence of both ovotestes and oviducts and the highest concentrations of vitellogenin. Moreover, the number of developing oocytes in the testes of the intersex fish (defined by the feminization index) was also the greatest in these fish.
Another important consideration is that, with the exception of the feminization index, the responses seen in the fish did not correlate with the total estrogenic activity present in the water samples as measured by the YES bioassay. Models of the interactions between the total estrogenic activity and the total antiandrogenic activity for each of the responses suggested that estrogenic components of the mixture sometimes appeared to antagonize or reduce responses in the fish that were associated with anti androgen exposure.
Discussion
These findings support the hypothesis that a combination of steroidal estrogens, nonylphenolic chemicals, and antiandrogens are most likely to cause widespread sexual disruption in wild fish populations in nature. By statistical modeling of the associations between each of the suspected causal factors and the suite of biological effects seen in fish, we established the likely influence of antiandrogens versus estrogens, both alone and in combination, on each response variable. Although these statistical analyses further support the role of steroidal estrogens in the causation of feminization of wild fish in U.K. rivers, they also suggest that antiandrogens are strong causal factors, necessary for severe effects to occur. Indeed, the likely influence of antiandrogenic chemicals on each of the measured responses is clearly demonstrated using a modeling strategy that allows for the effects of steroidal estrogens first before interrogating the data for the existence of additional causal factors. This approach further strengthens the hypothesis that feminization results from the effects of both antiandrogens and estrogens acting in concert.
Sometimes, the antiandrogens appear to act additively with the estrogens to increase a particular response (for oocytes and feminized ducts), whereas in other examples the effect of the antiandrogens appears greater than that of the estrogens (VTG in the blood plasma of males). For fem.duct, we found an interaction between the steroidal estrogens and antiandrogenic activity, the estrogens acting to decrease the response due to the antiandrogens. This does not necessarily imply that all of the factors were interacting to produce a particular response at the same time. Some of the responses (e.g., fem.duct) are induced during early development (e.g., Rodgers-Gray et al. 2001), whereas others (e.g., oocytes) manifest themselves throughout life (Jobling et al. 2006). It is conceivable that when additive relationships are seen, they could be the result of a concentration-related effect of an initiator (acting during early life) and a promoter (acting during adult life).
The estrogenic activity of the water samples (as measured in the YES bioassay) did not correlate well with any of the biological responses or with the concentrations of individual steroidal estrogens measured in the effluents. In most cases, the combined estrogenic activity of the steroidal estrogens present in the effluents was predicted to be higher than that actually measured using the YES bioassay. This lack of correlation between the YES assay results and the individual concentrations of steroidal estrogens could well have been due to the existence of antiestrogenic compounds in some of the effluents, which would reduce the response seen in the YES assay. Indeed the widespread existence of antiestrogenic benzotriazoles in STW effluents, which are potent in the YES bioassay, has recently been reported (Giger et al. 2006). Moreover, Harris et al. (2007) showed that benzotriazoles were not antiestrogenic in fish, even though they were potent anti estrogens in the YES bioassay, thus providing a possible explanation for the mismatch between the fish responses and the YES bio assay response. Indeed, the strong positive correlations of the biological responses with the steroidal estrogen concentrations but not the YES assay results add credence to this suggestion.
Although PCA indicated heterogeneity of antiandrogens and estrogens across sites, there were still correlations between some of the covariates, and the multicolinearity exhibited by these co-occurrent contaminants sometimes confounded the interpretation of the statistical analyses. For example, NP was always highly correlated with E1 (Table 2) and so its association with any of the biological effects could rarely be separated from that of E1. However, when the strength of the association between one of these parameters and a response was stronger than that of the other, it indicated that the former was a more likely cause than the latter. Intuitively, strong associations are more likely to be causal than weak ones (Hill 1965). Moreover, the statistical modeling strategy we adopted ensured that additional likely causal factors (anti androgenic components) were identified only after accounting for the effects of the main causal factors (steroidal estrogens).
Multicolinearity could also account for the possibility that none of the covariates were causes of feminization in wild fish and that they were masking the identity of an as yet unidentified chemical cause. In most cases, however, this possibility seems highly unlikely, as the association between the antiandrogenic activity and the responses would appear strong enough to rule out hypotheses that the associations are entirely due to one weak unmeasured confounder or other source of modest bias. Moreover, given the fact that laboratory experiments clearly show that exposure to antiandrogens (e.g., Kiparissis et al. 2003; Makynen et al. 2000) or steroidal or xenoestrogens (e.g., Seki et al. 2002; Yokota et al. 2001) can cause sexual disruption in fish, it seems plausible that chemicals with these mechanisms of action could also cause effects in wild fish. For example, inter sexuality and vitellogenin induction can be seen in fish exposed to concentrations of steroidal estrogens in the low nanograms-per-liter range. Moreover, at least with the vitellogenin response, combinations of steroidal (and other) estrogens have been shown to act additively to cause this effect (Brian et al. 2005; Thorpe et al. 2003).
As with estrogenic activity, antiandrogenic activity (given in flutamide equivalents) predicted to be present in the rivers was often sufficient to induce biological responses in fish (Katsiadaki et al. 2006; Kiparissis et al, 2003). In addition, molecular approaches studying changes in gene expression have shown that the feminizing effects of estrogens and antiandrogens in fish share both common and distinct gene pathways (Filby et al. 2007a, 2007b). It seems likely, therefore, that mechanisms exist by which combinations of estrogens and anti androgens could act together when they are administered in combination (Kortenkamp 2008), thus offering further support to some of the cause–effect associations postulated here.
These results clearly demonstrate that induced reproductive health effects in fish in U.K. rivers likely involve factors other than environmental estrogens. The results also provide an interesting parallel with the results of studies performed in rodent models to investigate the suspected environmental causation of testicular dysgenesis syndrome in humans, which is also thought to be mediated primarily by antiandrogenic combined with estrogenic mechanisms rather than by estrogenic mechanisms alone (Christiansen et al. 2008; Sharpe and Skakkebaek 2008; Skakkebaek et al. 2001; Wolf et al. 1999). Although analysis of the human data by itself has so far failed to provide firm evidence of direct causal associations between low-level exposure to specific endocrine-disrupting chemicals and endocrine disorders in humans, studies such as ours that link endocrine effects seen in wildlife to exposure to estrogens and anti androgens present in human domestic waste water may add further credence to the hypothesis that the effects seen in both wild fish and humans are caused by similar combinations of endocrine-disrupting chemical cocktails to which both fish and humans are exposed.
Footnotes
Supplemental Material is available online at http://www.ehponline.org/members/2009/0800197/suppl.pdf
Statistical modeling was supported by Beyond The Basics Ltd. and the U.K. Environment Agency. The U.K. National Survey of hormonal activity and chemical monitoring was sponsored by The U.K. Environment Agency. C.R.T. was further supported by the Natural Environment Research Council (NER/NE/E016634/1).
References
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Axelstad M, Boberg J, Kortenkamp A, Hass U. Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int J Androl. 2008;31(2):241–247. doi: 10.1111/j.1365-2605.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol. 1998;32:1549–1558. [Google Scholar]
- Environment Agency. Assessment of (Anti-) Oestrogenic and (Anti-) Androgenic Activities of Final Effluents from Sewage Treatment Works. Bristol, UK: Environment Agency; 2007. [[accessed 25 March 2009]]. Science Report SC020118/SR. Available: http://publications.environment-agency.gov.uk/pdf/SCHO0207BMAX-e-e.pdf. [Google Scholar]
- Filby AL, Santos EM, Thorpe KL, Maack G, Tyler CR. Gene expression profiling for understanding chemical causation of biological effects for complex mixtures: a case study on estrogens. Environ Sci Technol. 2007a;41:8187–8194. doi: 10.1021/es071278v. [DOI] [PubMed] [Google Scholar]
- Filby AL, Thorpe KL, Maack G, Tyler CR. Gene expression profiles revealing the mechanisms of anti-androgen- and estrogen-induced feminisation in fish. Aquat Toxicol. 2007b;81(2):219–231. doi: 10.1016/j.aquatox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models . Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Gibson R, Smith MD, Spary C, Tyler CR, Hill EM. Mixtures of oestrogenic contaminants in bile in fish exposed to wastewater treatment works effluents. Environ Sci Technol. 2005;39(8):2461–2471. doi: 10.1021/es048892g. [DOI] [PubMed] [Google Scholar]
- Giger W, Schaffner C, Kohler HPE. Benzotriazole and tolyl-triazole as aquatic contaminants. 1. Input and occurrence in rivers and lakes. Environ Sci Tech. 2006;40(23):7186–7192. doi: 10.1021/es061565j. [DOI] [PubMed] [Google Scholar]
- Harris CA, Routledge EJ, Schaffner C, Brian JV, Giger W, Sumpter JP. Benzotriazole is antiestrogenic in vitro but not in vivo. Environ Tox Chem. 2007;26:2367–2372. doi: 10.1897/06-587R.1. [DOI] [PubMed] [Google Scholar]
- Harries JE, Sheahan DA, Jobling S, Matthiessen P, Neall P, Sumpter JP, et al. Estrogenic activity in five United Kingdom rivers detected by measurement of vitellogenesis in caged male trout. Environ Toxicol Chem. 1997;16:534–542. [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc Royal Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998;32:2498–2506. [Google Scholar]
- Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, et al. Predicted exposures to steroid estrogens in UK rivers correlate with widespread sexual disruption in wild fish populations. Environ Health Perspect. 2006;114(suppl 1):32–39. doi: 10.1289/ehp.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Williams RJ, Simpson P, Kanda R. What difference might sewage treatment performance make to endocrine disruption in rivers? Environ Pollut. 2007;147(1):194–202. doi: 10.1016/j.envpol.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Katsiadaki I, Morris S, Squires C, Hurst MR, James JD, Scott AP. Use of the three-spined stickleback (Gasterosteus aculeatus) as a sensitive in vivo test for detection of environmental antiandrogens. Environ Health Perspect. 2006;114(suppl 1):115–121. doi: 10.1289/ehp.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Academy Sci USA. 2007;104(21):8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiparissis Y, Metcalfe TL, Balch GC, Metcalfe CD. Effects of the antiandrogens, vinclozolin and cyproterone acetate on gonadal development in the Japanese medaka (Oryzias latipes) Aquat Toxicol. 2003;63(4):391–403. doi: 10.1016/s0166-445x(02)00189-3. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl. 2008;31(2):233–237. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- Makynen EA, Kahl MD, Jensen KM, Tietge JE, Wells KL, Van Der Kraak G, et al. Effects of the mammalian antiandrogen vinclozolin on development and reproduction of the fathead minnow (Pimephales promelas). . Aquat Toxicol. 2000;48:461–475. doi: 10.1016/s0166-445x(99)00059-4. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London: Chapman & Hall; 1989. [Google Scholar]
- Nolan M, Jobling S, Sumpter JP, Brighty G, Tyler CR. A histological description of description of intersexuality in the roach (Rutilis rutilis) J Fish Biol. 2001;17:160–176. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing . Vienna: R Foundation for Statistical Computing; 2007. [[accessed 26 March 2009]]. Available: http://cran.r-project.org/doc/manuals/refman.pdf. [Google Scholar]
- Rodgers-Gray TP, Jobling S, Kelly C, Morris S, Brighty G, Waldock MJ, et al. Exposure of juvenile roach (Rutilus rutilus) to treated sewage effluent induces dose-dependent and persistent disruption in gonadal duct development. Environ Sci Technol. 2001;35:462–470. doi: 10.1021/es001225c. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, Sheahan D, Desbrow C, Brighty GC, Waldock M, Sumpter JP. Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ Sci Technol. 1998;32(11):1559–1565. [Google Scholar]
- Routledge EJ, Sumpter JP. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem. 1996;15:241–248. [Google Scholar]
- Seki M, Yokota M, Matsubara H, Tsuruda Y, Maeda M, Tadokoro H, et al. Effect of ethinylestradiol on the reproduction and induction of vitellogenin and testisova in medaka (Oryzias latipes) Environ Toxicol Chem. 2002;21(8):1692–1698. [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89(2 suppl):e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158(3):327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Thorpe K, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, et al. Relative potencies and combination effects of steroidal oestrogens in fish. Environ Sci Tech. 2003;37:1142–1149. doi: 10.1021/es0201348. [DOI] [PubMed] [Google Scholar]
- Thorpe K, Gross-Sorokin M, Johnson I, Brighty G, Tyler CR. An assessment of the model of concentration addition for predicting the estrogenic activity of chemical mixtures in wastewater treatment works effluents. Environ Health Perspect. 2006;114(suppl 1):90–97. doi: 10.1289/ehp.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda AM, Barber LB, Gray JL, Lopez EM, Woodling JD, Norris DO. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ Sci Technol. 2008;42(9):3407–3414. doi: 10.1021/es0720661. [DOI] [PubMed] [Google Scholar]
- Vethaak AD, Lahr S, Schrap M, Belfroid AC, Rijs GBJ, Gerritsen A, et al. An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere. 2005;59(4):511–524. doi: 10.1016/j.chemosphere.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Vos JG, Dybing E, Greim HA, Ladefoged O, Lambré C, Tarazona JV, et al. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit Rev Toxicol. 2000;30:71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15:94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- Yokota H, Asanori M, Seki M, Asanobu M, Aeda M, Uji Y, et al. Life-cycle toxicity of 4-nonylphenol to medaka (Oryzias latipes) Environ Toxicol Chem. 2001;20(11):2552–2560. doi: 10.1897/1551-5028(2001)020<2552:lctont>2.0.co;2. [DOI] [PubMed] [Google Scholar]