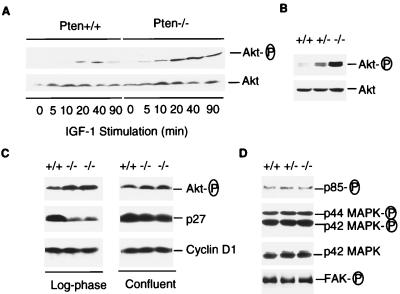
Figure 4.
Phosphorylation status of Akt, PI3 kinase, MAPK, and FAK, and the levels of p27 in Pten+/+, Pten+/−, or Pten−/− ES cells. (A) Phosphorylation status of Akt after IGF-I stimulation. Pten+/+ and Pten−/− ES cells were passed twice without feeders to reduced background. Cells were serum-starved for 34 hr, then stimulated by IGF-I (1 μg/ml) for indicated time periods. Cell lysates (25 μg each) were examined by Western blot analysis with antibodies against phospho-Akt (serine-473) or Akt, respectively. (B) Phosphorylation status of Akt in actively growing ES cells. Log-phase growing Pten+/+, Pten+/−, or Pten−/− ES cells were harvested, and the cell lysates were analyzed with antibodies against phospho-Akt or Akt, respectively. (C) Akt, p27KIP1, and cyclin D1 levels in cells from different proliferation states. Cells were harvested from either log-phase cultures (Left) or confluent cultures (Right). Cell lysates (50 μg each) were examined by Western blot analysis with antibody specific for phospho-Akt, p27, or cyclin D1, respectively. (D) Phosphorylation status of PI3 kinase, MAPK, and FAK. Cell lysates were prepared from log-phase growing cells. To determine the phosphorylation status of the p85 subunit of PI3 kinase and FAK, cell lysates (500 μg each) were immunoprecipitated with antiphosphotyrosine antibody 4G10 followed by Western blot analysis with anti-p85 or anti-FAK antibody, respectively. To detect phosphorylated p42 and p44 MAPK, cell lysates (50 μg each) were examined by Western blots analysis with an antibody against phospho-MAPK. As a control, a duplicate filter was analyzed in parallel with an antibody for p42 MAPK.