Table 1.
Parameters used in the population pharmacokinetic–pharmacodynamic (PK–PD) analysis for rabeprazole *
| Base PK model | Final PK model |
|---|---|
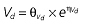 |
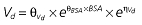 |
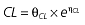 |
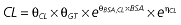 |
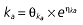 |
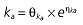 |
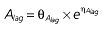 |
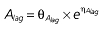 |
| Base PD model | Final PD model |
|---|---|
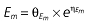 |
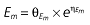 |
 |
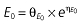 |
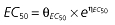 |
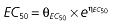 |
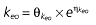 |
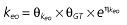 |
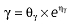 |
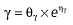 |
Vd, volume of distribution; CL, clearance; ka, absorption constant; Alag, absorption lagged time; Em, maximal effect; E0, baseline effect; EC50, concentration when half Em achieved; keo, elimination constant for the effect compartment; BSA, body surface area; γ, slope; GT, CYP2C19 genotype; θx, population mean of parameter x; ηx, intersubject variability of parameter x.
