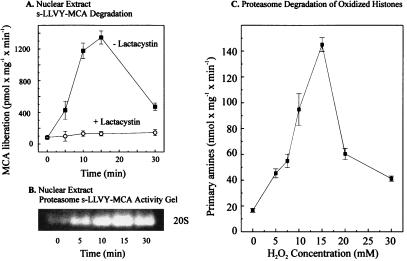
Figure 2.
Activation of nuclear proteolysis and increased proteolytic susceptibility of histones after hydrogen peroxide treatment. K562 cells were grown and exposed to H2O2 as described in the legend to Fig. 1. Cell nuclei then were isolated and lysed, and degradation of the fluorogenic peptide suc-LLVY-MCA was measured as described in Materials and Methods. (A) The lactacystin-sensitive suc-LLVY-MCA degradation as a function of time of K562 cell exposure to 1 mM hydrogen peroxide. The values represent the means of six independent measurements with SD less than 10%. (B) A representative suc-LLVY-MCA degradation activity stain of a one-dimensional polyacrylamide nondenaturing proteasome electrophoresis gel, according to ref. 21, also as a function of time of K562 cell exposure to 1 mM hydrogen peroxide. (C) The proteolytic susceptibility of H2O2-modified histones, incubated with the purified 20S proteasome. [3H]-labeled histones (at 2.0 mg protein/ml, Boehringer Mannheim) were radiolabeled and exposed (or sham-exposed) to hydrogen peroxide in vitro as described in Materials and Methods. The x axis shows the concentrations of hydrogen peroxide used to oxidize histones. After extensive dialysis, proteasome was added for a 2-h incubation, and proteolysis was measured by the fluorescamine assay for release of free amino acids, after incubation of 40 μg histone substrate protein with 0.6 μg proteasome (see Materials and Methods). All values in C represent the means ± SD of three independent experiments.