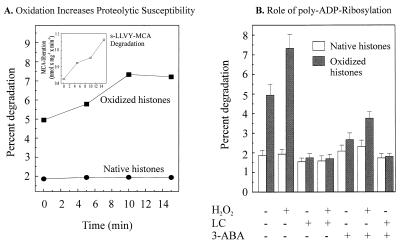
Figure 3.
Proteasome-dependent selective degradation of oxidized histones in nuclear extracts and the role of poly-ADP ribosylation. (A) The degradation of undamaged and H2O2-modified histones and suc-LLVY-MCA by lysates of H2O2-treated isolated nuclei from K562 hematopoietic cells. Nuclei were isolated, exposed to 1 mM H2O2 for times from 0 to 15 min (or sham exposed), lysed, and used to measure the proteolytic susceptibility of both untreated and H2O2-treated [3H]-labeled histones (added to the nuclear lysates) exactly as described in Materials and Methods. Isolated [3H]-labeled histones were oxidatively modified (or used as unmodified “native” histones) as described in Materials and Methods. The time scale represents the length of nuclei exposure (or sham-exposure) to H2O2 and the data points represent the means of three independent experiments (for which SD were always less than 10%). (Inset) The increase in the suc-LLVY-MCA degrading activity of nuclear lysates after treatment of nuclei with 1 mM H2O2 (for details see Fig. 2). (B) The degradation of H2O2-modified histones by K562 cell nuclear lysates is stimulated by pretreatment of nuclei with H2O2 but inhibited by both lactacystin and 3-ABA. Nuclei were isolated, exposed to 1 mM H2O2 for 15 min (or sham exposed), lysed, and used to measure the proteolytic susceptibility of both untreated and H2O2-treated [3H]-labeled histones, as described in Materials and Methods, in the presence or absence of the inhibitors 5 μM lactacystin (LC) or 1 mM 3-ABA. Where used, oxidized histones were treated with 15 mM hydrogen peroxide. All values represent the means ± SD of three independent experiments.