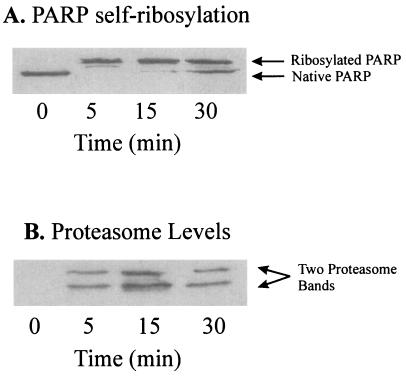
Figure 6.
Immunoprecipitation of the proteasome with a monoclonal anti-DBD-PARP antibody in nuclear extracts from H2O2-treated K562 cells. Intact K562 human chronic myelogenous leukemia cells were grown, harvested, treated with 1 mM H2O2, and then subjected to isolation and lysis of nuclei, as described in Materials and Methods. Immunoprecipitation was conducted with 100 μg of lysate protein, 100 μl of Tris/NaCl buffer (containing 50 mM Tris, plus 100 μM NaCl, at pH 8.0), and 1 μl of a mAb directed against the DBD (anti-DBD) of PARP (at 5 mg/ml), by a modification of the procedure of Zwilling et al. (30). The primary antibody reaction was performed in 50 mM Tris (pH 7.8) containing 150 mM NaCl (Tris buffered saline), 0.1% BSA, and 1.0 μl of rabbit-anti-DBD-IgG (5 μg/μl stock) for 1 h at 4°C in a total volume of 200 μl. For secondary antibody reactions 1.0 μl of donkey-anti-rabbit-IgG (5 μg/μl stock) was added and incubated for 1 h at 4°C. Binding of the secondary antibody was detected by using an ECL kit. Immunoprecipitation was initiated by addition of 20 μl of protein A-Sepharose (equilibrated 1:1 with a pH 8.0 solution of 50 mM Tris, 150 mM NaCl, and 0.1% BSA) for a 1-h incubation at 4°C. Controls were incubated with the secondary antibody and the protein A-Sepharose only. The Sepharose was centrifuged (12,000 × g for 30 min) and washed five times with Tris buffered saline. Sepharose-bound proteins were separated by 15% SDS/PAGE, transblotted onto nitrocellulose membranes, and incubated either with a polyclonal goat-anti-PARP-IgG (A) or a polyclonal rabbit-anti-core-proteasome-IgG (B). Control samples containing secondary antibody plus Sepharose A, or Sepharose A alone, produced no visible bands in the gels (data not shown). The results shown are a representative immunoblot from a series of several independent experimental repeats.