Abstract
Plants produce flowers with complex visual and olfactory signals, but we know relatively little about the way that signals such as floral scents have evolved. One important factor that may direct the evolution of floral signals is a pollinator's ability to learn. When animals learn to associate two similar signals with different outcomes, biases in their responses to new signals can be formed. Here, we investigated whether or not pollinators develop learned biases towards floral scents that depend on nectar reward quality by training restrained honeybees to learn to associate two similar odour signals with different outcomes using a classical conditioning assay. Honeybees developed learned biases towards odours as a result of differential conditioning, and the extent to which an olfactory bias could be produced depended upon the difference in the quality of the nectar rewards experienced during conditioning. Our results suggest that differences in reward quality offered by flowers influence odour recognition by pollinators, which in turn could influence the evolution of floral scents in natural populations of co-flowering plants.
Keywords: olfaction, sensory bias, honeybee, peak shift, pollinator, floral scent
1. Introduction
Flowering plants have evolved complex visual and olfactory signals as a means of advertising nectar rewards to attract and maintain the interest of pollinators (Dilcher 2001; Raguso 2004; Gang 2005). Many pollinators have concomitantly evolved the ability to learn to associate floral signals with nectar (Chittka & Thomson 2001; Chittka & Raine 2006). By learning which flowers offer the highest quality rewards, a pollinator can adapt rapidly to changes in food availability. It may also allow pollinators to avoid visiting flowers that offer lower quality nectar (Chittka & Schurkens 2001) or in which nectar is absent (Herrera et al. 2006) or even toxic (Detzel & Wink 1992; Adler 2000). Of particular importance during learning is a flower's scent; in fact, scent is often the critical cue used by a pollinator to recognize the type of flowers that it has previously successfully found associated with nectar (Kunze & Gumbert 2001; Raguso & Willis 2002, 2005; Salzmann et al. 2007). For example, bumble-bees are more likely to confuse unrewarding flowers with rewarding flowers when their scent signals are similar (Kunze & Gumbert 2001). They also learn to associate scented floral visual displays faster with nectar than visual displays without scent (Kulahci et al. 2008).
While it is clear that learning allows a pollinator to exploit a diverse ‘floral marketplace,’ what a pollinator learns about floral scent could also influence the way that it responds to newly encountered flowers as learning can bias an animal's response to signals and potentially drive the evolution of signaller–receiver relationships (Endler & Basolo 1998; ten Cate & Rowe 2007). For example, biases arise when a signal receiver learns to associate two similar signals with different outcomes: one with a positive outcome and the other with a negative outcome (Lynn et al. 2005; ten Cate et al. 2006; ten Cate & Rowe 2007). This form of bias (also called a ‘peak shift’) in an animal's response is characterized by larger responses towards novel but similar stimuli than towards the original, learned stimuli (Hanson 1959; for a review, see Ghirlanda & Enquist 2003). Novel stimuli that are not sufficiently similar to learned stimuli do not produce peak-shifted biases (Ghirlanda & Enquist 1999; ten Cate & Rowe 2007).
If a pollinator learns to associate two subtly different scent signals with nectar rewards of differing quality, a learned bias to respond towards newly encountered floral scents could be formed, which would affect its subsequent foraging behaviour and hence direct the evolution of floral scent. This expectation is not unreasonable, as the floral scents of populations of related plants often exhibit variation in subtle features such as differences in the concentration of the same odour compounds (Raguso et al. 2003; Wright & Thomson 2005; Wright et al. 2005), which result in small but perceivable differences (Wright et al. 2005, 2008). Furthermore, nectar rewards associated with floral scents also vary in quality (Petanidou et al. 2006) and quantity (Herrera et al. 2006) even in flowers produced by the same plant (Herrera et al. 2006). Nectar is the currency of the interaction between plants and their pollinators: it costs plants to produce nectar (Nepi & Stpiczynska 2008), and pollinators such as honeybees and bumble-bees carefully attend to the nectar quality and to the probability of encountering food offered by flowers (Waddington 2001; Cnaani et al. 2006; Drezner-Levy & Shafir 2007; Shafir et al. 2008). Thus, natural variation in both floral signals and floral rewards could result in pollinators with learned olfactory biases that could direct the evolution of floral scent signals in plant populations if these biases affected the transfer of conspecific pollen from one plant to another.
The series of experiments reported here was designed to address whether nectar quality affected the formation of a learned olfactory bias in the model insect pollinator, the honeybee. Using a classical conditioning assay developed to study differential olfactory learning (Bitterman et al. 1983), we trained individual honeybees to learn to associate two binary odours of different proportions of the same odorants (1-hexanol and 2-octanone) with different outcomes and then tested them to find whether a bias had been formed. These binary odours are perceptually similar to honeybees, but still distinguishable (Wright et al. 2008). Differences in the ratios of common components are also observed in closely related plant species (Raguso et al. 2003; Wright et al. 2005; Salzmann et al. 2007) and as such might be expected to be the stimulus feature to which a pollinator would attend to differentiate floral scents (Wright et al. 2005, 2008). As outcomes, we used appetitive rewards that simulated the potential differences in nectar quality that a honeybee might experience during foraging, including sucrose, proline (an amino acid), salt (NaCl), and the absence of reward. Proline is commonly found in the floral nectar of honeybee-pollinated plant species (Petanidou et al. 2006), and it was used here to enhance the attractiveness of a reward based on a previous experiment, which showed that honeybees preferred sucrose solutions containing 0.01 M proline over sucrose solutions alone (Carter et al. 2006). Salt and ‘no reward’ were used as negative reinforcers because ions such as potassium and sodium can repel pollinators in high concentrations when found in nectar (Waller et al. 1972; Nicolson & Worswick 1990; Afik et al. 2006a,b). Furthermore, flowers not containing nectar (Drezner-Levy & Shafir 2007; Salzmann et al. 2007) or offering nectar with a low flow rate (Greggers & Menzel 1993) are also important for pollinators to learn to avoid visiting. Nectar sugar concentration is often variable (Herrera et al. 2006) and affects pollinator preferences in natural settings (Chittka & Schurkens 2001), so we also examined whether simple differences in the amount of sucrose present in a reward could bias a honeybee towards more rewarding floral scents.
2. Material and methods
(a) Subjects
Worker honeybees (Apis mellifera carnica) were collected and restrained as described in Wright & Smith (2004). Each subject was fed to satiety (20–30 μl) with 1.0 M sucrose and left on the bench at room temperature for approximately 24 hours before conditioning. At least 10 min before an experiment, the antenna of each subject was stimulated with a droplet of sucrose solution to provoke the proboscis extension reflex; if a subject did not respond by extending its proboscis, it was not used in the experiments.
(b) Odour stimuli
The odour compounds used in our experiments were 1-hexanol and 2-octanone (99.8% purity; Sigma-Aldrich, St Louis, MO); both of these volatile compounds are found in floral scents (Knudsen et al. 2006), are perceptually distinctive to honeybees (Wright & Smith 2004) and have been used in several previous investigations of honeybee olfactory learning (Wright & Smith 2004; Wright et al. 2005, 2008). These odours were mixed as proportions from a stock solution of 0.02 M; the original, neat odour solutions were diluted in mineral oil to obtain the specific molar concentration. Our stimulus set was prepared such that the proportions of 1-hexanol (ph) in each mixture were 0.1, 0.3, 0.5, 0.7 and 0.9 in a final volume of 20 ml in a 60 ml Boston bottle. The top of the Boston bottle was stopped with a silicon cork into which two 26-gauge syringe needles had been inserted to facilitate air flow in and out of the bottle's headspace as described in Brown et al. (2005). Silicon tubing was attached, using plastic fittings, to each syringe needle head; one tube was connected to the valve controlled by the conditioning apparatus and the other was aimed at the subject in the conditioning arena. The first discharge of the odour stimulus headspace was aimed into the air exhaust of the conditioning arena 30 s prior to conditioning. The odour stimuli were presented as 4 s pulses at an inter-trial interval of 10 min using an apparatus described in Wright & Smith (2004); each pulse was composed of air containing volatilized odour of the headspace of the bottle produced from the flow rate of 40 ml s−1. Each stimulus was presented after approximately 20–30 s had passed, allowing a headspace to develop in the bottle, which was determined by the time between stimuli and which was regular across subjects and conditioning trials.
(c) Conditioning and testing
Individual, restrained worker honeybees were trained to extend the proboscis (mouthparts) when presented with an odour associated with a food reward using conditioning techniques described in Bitterman et al. (1983). Two different techniques were used: simple conditioning, in which one odour stimulus was paired with a 1.0 M sucrose reward on every trial for 16 trials; and differential conditioning, in which two odours were conditioned on pseudorandomly alternating trials, such that each odour was associated with a different outcome as reported in Wright et al. (2008). For the simple conditioning, the odour used as the conditioned stimulus (CS) was always the ph=0.5. For the differential conditioning, the ‘positively reinforced’ stimulus (CS+) was always ph=0.5, while the negatively reinforced stimulus (CS−) was always ph=0.7. As a control, we tested whether the response function generated by differential conditioning was symmetric about CS+ of ph=0.5 by differentially conditioning a separate group of subjects with CS− of ph=0.3. We found no significant difference in the response functions produced by differential conditioning with CS− of ph=0.7 or ph=0.3 (logistic regression, lreg, two-way interaction: Χ12=1.46, p=0.225; N0.3=30, N0.7=33), indicating that the olfactory response function did not depend on the odour used as the CS− (see the electronic supplementary material.)
(d) Experiment 1: can olfactory biases be formed as a result of differential learning?
In this experiment, we examined whether it was possible to produce a learned bias or ‘peak-shift’ towards a novel odour stimulus. In the control treatment (CS+ only), honeybees experienced simple conditioning with the ph=0.5 odour in association with a 0.4 μl droplet of 1.0 M sucrose solution. In the peak-shift treatment (CS+/CS−), honeybees experienced differential conditioning where the ph=0.5 odour was reinforced with 1.0 M sucrose solution and the ph=0.7 odour was reinforced with 1.0 M NaCl solution applied to the antennae. Both the sucrose and NaCl solutions were delivered using separate Gilmont micrometre syringes. After both types of conditioning, each honeybee was presented with the five odour stimuli (ph=0.1, 0.3, 0.5, 0.7, 0.9) in five unreinforced test trials; the order of the tests with each odour were randomized across subjects.
(e) Experiment 2: does the strength of an olfactory bias depend on the relative difference in the reinforcers?
This experiment was designed to examine how the difference in the positive and negative reinforcers (i.e. nectar rewards of different qualities) influenced the peak-shift formed as a result of differential conditioning. Three treatments using the same differential conditioning method described in experiment 1 were designed to examine this in a manner that simulated potential differences in nectar qualities that a honeybee might encounter while foraging. Reinforcers in each treatment were delivered using separate Gilmont micrometre syringes. In the ‘large difference in nectar quality’ treatment, the ph=0.5 odour was positively reinforced with a 1.0 M sucrose solution containing 0.01 M of an amino acid, proline, and the ph=0.7 was negatively reinforced with a 1.0 M NaCl solution applied to the antennae. In the ‘moderate difference in nectar quality’ treatment, the ph=0.5 odour was positively reinforced with a 1.0 M sucrose solution and the ph=0.7 received no reinforcement, simulating a situation in which a honeybee visited an unrewarding flower. In the ‘small difference in nectar quality’ treatment, the ph=0.5 odour was positively reinforced with a 1.0 M sucrose solution and the ph=0.7 was negatively reinforced with a 0.3 M sucrose solution, simulating the situation in which a honeybee is confronted with two flowers offering different qualities of reward. These sucrose solutions were chosen based on previous work which showed that honeybees could detect differences in their concentrations (Page et al. 1998). After all three treatments, each honeybee was presented with the same five odour stimuli described in experiment 1 (ph=0.1, 0.3, 0.5, 0.7, 0.9) in five unreinforced test trials; tests with each odour were randomized across subjects.
(f) Data analysis
During each test period after conditioning, the response variable measured was whether a honeybee extended its proboscis in response to an odour (a binary variable, yes or no). Binary lreg was used, therefore, to analyse response probabilities to each test odour and to compare gradients formed by each treatment (SAS software, PROC GENMOD). One-tailed least-squares multiple comparisons (LSMC) were conducted to make specific pairwise comparisons among test odours.
3. Results
(a) Differential learning produces an olfactory bias towards novel odours
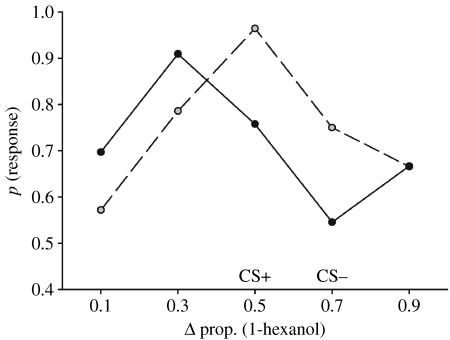
If honeybees experienced two different outcomes associated with perceptually similar olfactory stimuli (e.g. ph=0.5 and ph=0.7), they exhibited a bias in their responses towards the test odours in the olfactory gradient that was shifted towards novel odours similar to the CS+ and further away in the gradient from the CS− (e.g. ph=0.3; figure 1). This bias was generated by the honeybees' experiences during appetitive learning and was not a result of a pre-existing sensory bias towards these stimuli, as it was not observed if honeybees had been conditioned for the same number of trials with only the CS+ (ph=0.5). When honeybees were conditioned with the CS+ only (ph=0.5), the test odour stimulus that elicited the greatest response was the CS+; the other test odours in the gradient elicited a response in a honeybee in a manner that was proportional to the differences in ratios between the test odour and the CS+.
Figure 1.
A comparison of the olfactory response function generated by associating two closely related odours with different outcomes (solid line, CS+/CS−, CS+=1.0 M sucrose and CS−=1.0 M salt, N=33). The response function formed simply by associating one odour with 1.0 M sucrose (dashed line, CS+, N=28) reveals that differential learning produces a bias towards novel olfactory stimuli (lreg, two-way interaction Χ22=7.03, p=0.030). For each response function, the ‘peak’ was significantly different from all other points along the gradient (comparisons performed within a gradient to the peak point only, one-tailed LSMC: p<0.05). The x-axis refers to the composition of the binary odour presented during the test; ph refers to the proportion of 1-hexanol (ph) present in the binary odour. The CS+ was always ph=0.5 and the CS− was always ph=0.7. The y-axis represents the probability of observing a conditioned response (proboscis extension) by honeybees towards a test odour.
(b) The strength of an olfactory bias depends upon the difference in the learned outcomes
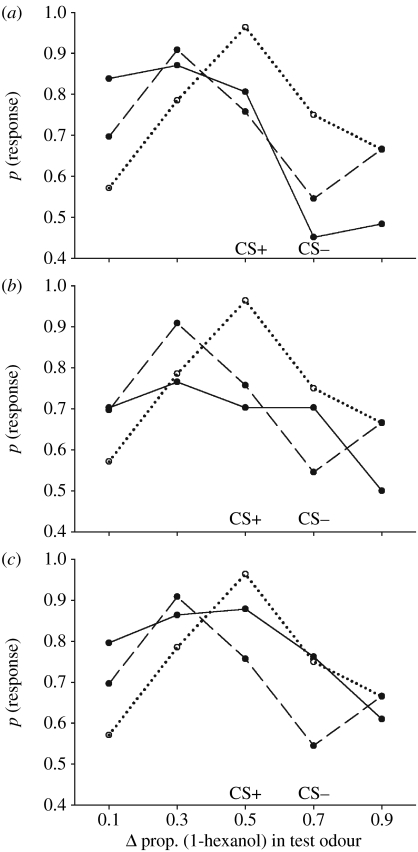
This experiment was performed to compare how the relative difference in the outcomes associated with odours during differential learning affected the expression of an olfactory bias (figure 2). Three pairs of outcomes that simulated situations a honeybee might encounter while foraging for nectar were differentially conditioned with a positively reinforced CS (ph=0.5) and a negatively reinforced CS (ph=0.7) and then tested with the same series of test stimuli as in experiment 1. The responses of honeybees to the test odours in these conditions were compared to those depicted in figure 1. When honeybees were tested with the five olfactory stimuli of varying proportions comprising the olfactory gradient, we observed that the pair of outcomes with the greatest difference (CS+ with 1.0 M sucrose and 0.01 M proline, CS− with 1.0 M NaCl; figure 2a) produced the largest bias; in this case, the olfactory response function was highly skewed away from the CS− and towards the CS+ with the highest responses elicited by novel odours similar to the CS+ and further away from the CS−. The shape of this response function was not significantly different from that produced by differential conditioning with the CS+/CS− (sucrose/NaCl) in figure 1 (lreg, two-way interaction: Χ42=4.79, p=0.310), but it was significantly different from that produced by the CS+ (sucrose only) conditioning, (lreg, two-way interaction: Χ42=16.9, p=0.002). To examine whether a ‘peak’ was formed in the sucrose–proline/NaCl response function, LSMC were performed for the peak (ph=0.3) against all other test odours in the gradient; the CS+ was significantly different (p<0.050) from the following test odours (indicated by the ph): 0.7 and 0.9.
Figure 2.
The relative difference between outcomes experienced during differential conditioning affected the strength of a learned, olfactory bias. The graphs are comparisons of the olfactory response functions towards a gradient of binary odours produced during a test period after conditioning with two perceptually similar binary odours associated with different outcomes. In each graph, the dotted/dashed lines indicate the response functions from figure 1 (dotted line= CS+1.0 M sucrose and CS−1.0 M NaCl; dashed line=CS+1.0 M sucrose only); the axes are also the same as those in figure 1. The solid lines in each graph represent the response functions produced by differential conditioning with: (a) CS+ reinforced with 1.0 M sucrose containing 0.01 M proline and the CS− reinforced with 1.0 M salt (N=31); (b) the CS+ reinforced with 1.0 M sucrose and the CS− without reinforcement (CS−) (N=64); and (c) the CS+ reinforced with 1.0 M sucrose and the CS− reinforced with 0.3 M sucrose (N=59).
When honeybees experienced a differential conditioning such that one odour was reinforced with 1.0 M sucrose and the other reinforced with ‘no reward’, the olfactory response function was also skewed towards the CS+ and away from the CS−, but the effect was much weaker than that produced by the sucrose/NaCl and sucrose–proline/NaCl conditioning (figure 2b). Unlike any of the other response functions in figure 2, it contained no obvious ‘peak’. This response function was significantly different from that produced by the CS+ only conditioning (lreg, two-way interaction: Χ42=12.1, p=0.017). It was not significantly different from that produced by the sucrose/NaCl conditioning (lreg, two-way interaction: Χ42=7.57, p=0.109), though the response at the peak of the sucrose/NaCl function (ph=0.3) was significantly higher (p=0.045) than the same point in the sucrose/no reward function. Because there was no obvious peak in the sucrose/no reward function, multiple comparisons were performed for the CS+ (ph=0.5) against all other test odours; the CS+ was significantly different only from the 0.9 test odour (p=0.020).
If honeybees had been conditioned with two different sucrose solutions (CS+ reinforced with 1.0 M sucrose CS− with 0.3 M sucrose; figure 2c), the olfactory response function did exhibit a peak, but this peak was not shifted away from the CS+. Because the peak was centred on the CS+ instead of being shifted away from the CS−, the shape of the high/low sucrose response function was not significantly different from that produced by conditioning the CS+ alone (lreg, two-way interaction: Χ42=7.92, p=0.094). Conditioning with two sucrose solutions also produced a slight skew in the generalization gradient towards the higher concentration of sucrose, such that the response to the left-most endpoint of the gradient (ph=0.1) was significantly higher for the high/low sucrose subjects than for the CS+ only subjects. The peak of the high/low sucrose gradient (ph=0.5) was compared with the responses at all other points in the gradient and, unlike the response function produced by the CS+ only conditioning, only one point in the gradient was significantly different (ph=0.9, p=0.002). The sucrose/sucrose gradient was also not significantly different from the sucrose/NaCl gradient (lreg, two-way interaction: Χ42=5.85, p=0.210). A direct comparison of each point in the gradient between the two response functions revealed that only the response to the CS− was significantly different (figure 2c, ph=0.7, p=0.034).
4. Discussion
Our experiments show that olfactory biases can be formed towards complex novel odours as a result of learning to associate two perceptually similar odours with different outcomes. When a honeybee was differentially conditioned with a binary odour associated with 1.0 M sucrose and another binary odour composed of different proportions of the same two odour compounds associated with 1.0 M NaCl, its responses towards novel proportions of the binary odour were biased towards the positively reinforced odour and away from the negatively reinforced odour in an olfactory gradient. This response function is characteristic of the peak shift formed as a result of differential learning observed in other modalities (Hanson 1959; Ghirlanda & Enquist 2003; Lynn et al. 2005; ten Cate & Rowe 2007). The magnitude of the peak-shifted bias depended upon the relative difference in the outcomes associated with odours: honeybees experiencing a large difference in reward quality during differential conditioning exhibited the greatest olfactory bias. Thus, olfactory biases can be formed when honeybees learn to associate two similar odours with different nectar rewards, and the strength of the bias depends upon the relative difference in the quality of the two rewards.
(a) Olfactory signals and learned olfactory biases
Most naturally occurring olfactory stimuli are complex mixtures of several odorant compounds emitted in unequal proportions (Wright & Thomson 2005). In fact, the floral scents of related plant species often possess the same scent compounds with the only difference being the proportions of each compound (Raguso et al. 2003; Wright et al. 2005). To understand whether olfactory receiver biases could evolve in a natural setting, it was important first to establish whether a bias could be formed towards the features of complex odours such as the ratios of two odorants in a complex mixture (Wright et al. 2005, 2008). In our experiments, when honeybees experienced differential conditioning such that a binary odour of specific proportions was reinforced with sucrose reward and another binary odour with different proportions of the same compounds was reinforced with a salt ‘punishment’, an olfactory bias (peak shift) was formed in a stimulus gradient composed of different proportions of the same binary mixture. Specifically, honeybees responded mostly to a binary odour that was a ph=0.2 to the left of the positively rewarded odour and ph=0.4 to the left of the negatively punished odour in an olfactory gradient. Though previous studies have observed less dramatic changes or ‘area shifts’ (ten Cate & Rowe 2007) towards novel, but similar odours as a result of differential olfactory learning (Daly et al. 2001; Wright et al. 2008), our experiments are the first to show that a bias, or a response that is stronger towards a novel stimulus than the learned stimulus, can be formed as a result of differential conditioning with olfactory stimuli.
The shape of this biased response function has classically been observed after differential conditioning with visual or auditory stimuli, which vary with respect to qualitative characteristics such as colour or tone (for reviews see: Ghirlanda & Enquist 1999, 2003; ten Cate & Rowe 2007). For example, when pigeons are trained to peck for food in response to coloured light stimuli after differential conditioning with two similar wavelengths (550 vs 570 nm), their responses towards a gradient of test stimuli of different wavelengths becomes ‘peak-shifted’ (Hanson 1959). Learned biases have also been observed in natural tasks performed by birds; a young male zebra finch prefers to mate with females that have a beak colour similar to that exhibited by its mother and avoid birds with beaks coloured similarly to its father's when presented with a variety of potential mates with a gradient of beak colours. If the beaks of its mother and father are similar in colour, the courtship response of a young male towards novel females will be peak-shifted in the direction of the colour of his mother's beak (ten Cate et al. 2006; ten Cate & Rowe 2007).
When animals are tested with stimulus parameters that vary along a qualitative dimension such as colour, the response functions resulting from differential learning typically follow a ‘rearrangement’ form, such as a peak-shifted response function, rather than a simple monotonic gradient observed for intensity-based changes in a stimulus (Ghirlanda & Enquist 2003). If the differences in ratio that we used in our experiments produced differences in the intensity of the stimulus only, we might expect simply to observe a monotonic function after differential conditioning rather than a rearrangement function. The fact that we observe a rearrangement function such as that demonstrated for a pigeon's response to a coloured light stimulus (Hanson 1959) or that of a bumble-bee to coloured, artificial flowers (Lynn et al. 2005) may indicate that honeybees perceive differences in ratio of specific compounds in a complex odour as differences in the qualitative properties of odours. This is supported by the fact that studies with humans have shown that differences in the ratio of two odours in a binary mixture affect an odour's perceptual qualities (Olsson & Cain 2000; Wise et al. 2000).
(b) Peak shift depends upon the relative difference in the reinforcers
Peak shift is observed in generalization gradients under a limited set of conditions that depend in part on the characteristics of learned stimuli (for reviews see Ghirlanda & Enquist 1999, 2003). The probability that an animal will generalize a learned response to a new stimulus depends on the perceptual similarity of the new and learned stimuli, and generalization along a stimulus gradient typically follows a Gaussian or exponential function (Shepard 1987). Differential learning with two stimuli, one of which is excitatory and the other inhibitory, does not produce a Gaussian generalization function, however (Hull 1943; Mackintosh 1974; Blough 1975; Ghirlanda & Enquist 1999). Instead, a peak-shifted generalization function is often formed, which may result from ‘two opposed response tendencies’—an excitatory function and an inhibitory one (Spence 1937; Mackintosh 1974; Blough 1975)—produced by learning to associate two similar conditioned stimuli with two different unconditioned stimuli (US). The neural mechanisms mediating the formation of peak-shifted generalization gradients are largely unknown, however.
The production of peak shift in generalization gradients may also be influenced by characteristics of the reinforcers. For example, previous studies have shown that the associative strength of the CS–US contingency, influenced by either the amount of conditioning or the relative frequency of positively and negatively reinforced trials (Lynn et al. 2005), influences peak shift (McLaren & Mackintosh 2002). As the associative strength of a CS–US contingency is mainly derived from the relative salience of both the CS and US (Rescorla 1976; Pearce 1994), we expected that the magnitude of the difference in the stimuli used as the positive and negative reinforcers (US; i.e. the potential difference in nectar quality that a foraging honeybee might encounter) should also affect the expression of olfactory peak shift. As predicted, the relative difference in the salience of the reinforcers used during differential conditioning influenced peak shift: we only observed a peak-shifted response function when a honeybee experienced a large difference in the salience of the positive and negative reinforcers. Furthermore, when the outcomes associated with two CS are positive and equal in salience, then the gradient produced after associative conditioning is flattened relative to the CS+ condition alone (Wright et al. 2008). Thus, our results confirm that the difference in the associative strength of the CS–US contingencies experienced during differential conditioning is fundamental to the production of peak shift in generalization gradients.
(c) Biases caused by differences in reward quality may drive the evolution of floral scent
Floral scent signals and floral rewards vary substantially in the populations of flowering plants that constitute a pollinator's ‘marketplace’, and a pollinator must continually update its information about the relationship between a floral signal and the availability and quality of floral resources. Any aspect of a floral signal that predicts a difference in the reward offered could improve a pollinator's efficiency in locating the most profitable resources prior to their exploitation by other pollinators. Furthermore, while differences in the proportion of two odours in the complex scent used in our experiments were subtle, the honeybees in our experiments could distinguish these odours, as observed in previous studies (Wright et al. 2005, 2008). That bees can detect such small differences in the quantities of odour compounds in complex floral scents suggests that they can exert selection pressure on the quantities of these compounds in floral signals. Thus, an olfactory bias produced by differences in reward quality could have an impact on the evolution of scent signals in populations of flowering plants competing for the attentions of the same pollinators if coexisting plants in a ‘floral marketplace’, possessing similar but distinguishable scents (e.g. flowers with different proportions of the same odour compounds), offer unequally valued rewards.
Our data support the idea that pollinators attend to differences in reward quality and rapidly adjust what they know about the resources associated with floral scent signals (i.e. the CS–US contingency), as the relative difference in reinforcement associated with two odour signals changed the way that a honeybee responded to similar odours. In particular, a strong bias in the response function was produced by simply tasting a salt solution associated with odour. Studies with free-flying honeybees which report that solutions containing toxic substances (Detzel & Wink 1992; Adler 2000; London-Shafir et al. 2003; Singaravelan et al. 2005) or with high concentrations of ions such as potassium or sodium are often repellent (Afik et al. 2006a,b) support the idea that a bad-tasting solution is a highly salient, negative reinforcer for a honeybee. Our results imply that if honeybees experience toxic or repellent nectar while foraging, other co-flowering plants without these substances could easily draw pollinators away because of the contrast in reward quality (Nicolson & Worswick 1990; Afik et al. 2006b). Concomitantly, if a negative bias away from floral traits associated with toxins in nectar was formed, plants with toxins could also benefit from this, as generalist pollinators less efficient at spreading pollen to conspecific plants would avoid visiting them. This would preserve the plant's nectar rewards so that more nectar would be left for efficient, specialist visitors unaffected by the toxin (Adler 2000; Kessler & Baldwin 2007).
If the olfactory biases exhibited by honeybees in our experiments reflect how a pollinator's foraging preferences are biased by its experience, these biases could influence plant fitness. Models of signal evolution suggest that even a slight bias or ‘skew’ towards a scent associated with a better reward (as observed in our experiments, where the negative reinforcer was either no reward or 0.3 M sucrose) could select for signallers biased towards the signal associated with the positive reinforcer and away from the signal associated with the negative reinforcer (ten Cate & Rowe 2007). Thus, an imbalance in reward could result in selection for plants that produce flowers with scents similar to the most rewarding co-flowering plants in a particular population and away from signals produced by plants with relatively poorer rewards. As a result of the potential for producing such biases, plants could be under pressure to maintain rewards of similar quality to neighbouring, co-flowering plants, even when doing this is costly. Nutrients such as carbohydrates and amino acids are expensive for a plant to produce as a reward, and nectar that is not collected by pollinators is often resorbed (Nepi & Stpiczynska 2008). Amino acids may be even more costly than sugars because they contain nitrogen, a growth-limiting nutrient for plants (Epstein & Bloom 2005). When differences in reward quality experienced by a pollinator are large, as when highly attractive substances such as proline are added to a sucrose reward (Carter et al. 2006), a strong, skewed olfactory bias with an extended peak shift can be produced. It is possible that even though amino acids are more metabolically costly to plants than sugars, their near-ubiquitous presence in floral nectar (Baker & Baker 1983; Gardener & Gillman 2002; Carter et al. 2006; Petanidou et al. 2006) could arise from the need to compete with other co-flowering plants for access to pollinators, and, in particular, pollinators that have co-evolved the ability to form learned biases towards floral signals that predict higher quality rewards.
Acknowledgements
The authors thank Malcolm B. Thompson for providing honeybee colonies, Candy Rowe and Carel ten Cate for conversations that led to these experiments, and Mitchell Thomson, Candy Rowe and three anonymous reviewers for comments on earlier versions of the manuscript. This research was funded in part by a Nuffield Foundation Science Research Bursary for M.A.B.
Supplementary Material
Symmetry of peak shift in an olfactory gradient
References
- Adler L.S. The ecological significance of toxic nectar. Oikos. 2000;91:409–420. doi:10.1034/j.1600-0706.2000.910301.x [Google Scholar]
- Afik O., Dag A., Kerem Z., Shafir S. Analyses of avocado (Persea americana) nectar properties and their perception by honey bees (Apis mellifera) J. Chem. Ecol. 2006a;32:1949–1963. doi: 10.1007/s10886-006-9120-1. doi:10.1007/s10886-006-9120-1 [DOI] [PubMed] [Google Scholar]
- Afik O., Dag A., Shafir S. The effect of avocado (Persea americana) nectar composition on its attractiveness to honey bees (Apis mellifera) Apidologie. 2006b;37:317–325. doi: 10.1007/s10886-006-9120-1. doi:10.1051/apido:2005064 [DOI] [PubMed] [Google Scholar]
- Baker H.G., Baker I. Floral nectar sugar constituents in relation to pollinator type. In: Jones C.E., Little R.J., editors. Handbook of experimental pollination biology. Van Nostrand Reinhold; New York, NY: 1983. pp. 117–141. [Google Scholar]
- Bitterman M.E., Menzel R., Fietz A., Schafer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J. Comp. Psychol. 1983;97:107–119. doi:10.1037/0735-7036.97.2.107 [PubMed] [Google Scholar]
- Blough D.S. Steady state data and a quantitative model of operant generalization and discrimination. J. Exp. Psych. Anim. Behav. Proc. 1975;104:3–21. doi:10.1037/0097-7403.1.1.3 [Google Scholar]
- Brown S.L., Joseph J., Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nat. Neurosci. 2005;8:1568–1576. doi: 10.1038/nn1559. doi:10.1038/nn1559 [DOI] [PubMed] [Google Scholar]
- Carter C., Shafir S., Yehonatan L., Palmer R.G., Thornburg R. A novel role for proline in plant floral nectars. Naturwissenschaften. 2006;93:72–79. doi: 10.1007/s00114-005-0062-1. doi:10.1007/s00114-005-0062-1 [DOI] [PubMed] [Google Scholar]
- Chittka L., Raine N.E. Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 2006;9:428–435. doi: 10.1016/j.pbi.2006.05.002. doi:10.1016/j.pbi.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Chittka L., Schurkens S. Successful invasion of a floral market—an exotic asian plant has moved in on europe's river-banks by bribing pollinators. Nature. 2001;411:653. doi: 10.1038/35079676. doi:10.1038/35079676 [DOI] [PubMed] [Google Scholar]
- Chittka L., Thomson J.D. Cambridge University Press; Cambridge, UK: 2001. Cognitive ecology of pollination: animal behaviour and floral evolution. [Google Scholar]
- Cnaani J., Thomson J.D., Papaj D.J. Flower choice and learning in foraging bumblebees: effects of variation in nectar volume and concentration. Ethology. 2006;112:478–485. doi:10.1111/j.1439-0310.2006.01174.x [Google Scholar]
- Daly K.C., Chandra S., Durtschi M., Smith B.H. The generalization of an olfactory-based conditioned response reveals unique but overlapping odour representations in the moth Manduca sexta. J. Exp. Biol. 2001;203:3085–3095. doi: 10.1242/jeb.204.17.3085. [DOI] [PubMed] [Google Scholar]
- Detzel A., Wink M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology. 1992;4:8–18. doi:10.1007/BF01245891 [Google Scholar]
- Dilcher D.L. Paleobotany: some aspects of non-flowering and flowering plant evolution. Taxon. 2001;50:697–711. doi:10.2307/1223702 [Google Scholar]
- Drezner-Levy T., Shafir S. Parameters of variable reward distributions that affect risk sensitivity of honey bees. J. Exp. Biol. 2007;210:269–277. doi: 10.1242/jeb.02656. doi:10.1242/jeb.02656 [DOI] [PubMed] [Google Scholar]
- Endler J.A., Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. doi:10.1016/S0169-5347(98)01471-2 [DOI] [PubMed] [Google Scholar]
- Epstein E., Bloom A.J. Sinauer; Sunderland, MA: 2005. Mineral nutrition of plants: principles and perspectives. [Google Scholar]
- Gang D.R. Evolution of flavors and scents. Annu. Rev. Plant Biol. 2005;56:301–325. doi: 10.1146/annurev.arplant.56.032604.144128. doi:10.1146/annurev.arplant.56.032604.144128 [DOI] [PubMed] [Google Scholar]
- Gardener M.C., Gillman M.P. The taste of nectar—a neglected area of pollination ecology. Oikos. 2002;98:552–557. doi:10.1034/j.1600-0706.2002.980322.x [Google Scholar]
- Ghirlanda S., Enquist M. The geometry of stimulus control. Anim. Behav. 1999;58:695–706. doi: 10.1006/anbe.1999.1187. doi:10.1006/anbe.1999.1187 [DOI] [PubMed] [Google Scholar]
- Ghirlanda S., Enquist M. A century of generalisation. Anim. Behav. 2003;66:15–36. doi:10.1006/anbe.2003.2174 [Google Scholar]
- Greggers U., Menzel R. Memory dynamics and foraging strategies of honeybees. Behav. Ecol. Sociobiol. 1993;32:17–29. doi:10.1007/BF00172219 [Google Scholar]
- Hanson H. Effects of discrimination training on stimulus generalization. J. Exp. Psychol. 1959;58:321–333. doi: 10.1037/h0042606. doi:10.1037/h0042606 [DOI] [PubMed] [Google Scholar]
- Herrera C.M., Perez R., Alonso C. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. Am. J. Bot. 2006;93:575–581. doi: 10.3732/ajb.93.4.575. doi:10.3732/ajb.93.4.575 [DOI] [PubMed] [Google Scholar]
- Hull C.L. Principles of behavior. Appleton-Century-Crofts; New York, NY: 1943. [Google Scholar]
- Kessler A., Baldwin I.T. Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant Journal. 2007;49:840–854. doi: 10.1111/j.1365-313X.2006.02995.x. doi:10.1111/j.1365-313X.2006.02995.x [DOI] [PubMed] [Google Scholar]
- Knudsen J.T., Eriksson R., Gershenzon J., Stahl B. Diversity and distribution of floral scent. Botan. Rev. 2006;72:1–120. doi:10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2 [Google Scholar]
- Kulahci I.G., Dornhaus A., Papaj D.R. Multimodal signals enhance decision making in foraging bumble-bees. Proc. R. Soc. B. 2008;275:797–802. doi: 10.1098/rspb.2007.1176. doi:10.1098/rspb.2007.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze J., Gumbert A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 2001;12:447–456. doi:10.1093/beheco/12.4.447 [Google Scholar]
- London-Shafir I., Shafir S., Eisikowitch D. Amygdalin in almond nectar and pollen—facts and possible roles. Plant Syst. Evol. 2003;238:87–95. [Google Scholar]
- Lynn S.K., Cnaani J., Papaj D.R. Peak shift discrimination learning as a mechanism of signal evolution. Evolution. 2005;59:1300–1305. doi:10.1554/04-284 [PubMed] [Google Scholar]
- Mackintosh N. The psychology of animal learning. Academic Press; London, UK: 1974. [Google Scholar]
- McLaren I.P.L., Mackintosh N.J. Associative learning and elemental representation: II. Generalization and discrimination. Anim. Learn. Behav. 2002;30:177–200. doi: 10.3758/bf03192828. [DOI] [PubMed] [Google Scholar]
- Nepi M., Stpiczynska M. The complexity of nectar: secretion and resorption dynamically regulate nectar features. Naturwissenschaften. 2008;95:177–184. doi: 10.1007/s00114-007-0307-2. doi:10.1007/s00114-007-0307-2 [DOI] [PubMed] [Google Scholar]
- Nicolson S.W., Worswick P.V.W. Sodium and potassium concentrations in floral nectars in relation to foraging by honey-bees. S. Afr. J. Zool. 1990;25:93–96. [Google Scholar]
- Olsson M.J., Cain W.S. Psychometrics of odor quality discrimination: method for threshold determination. Chem. Senses. 2000;25:493–499. doi: 10.1093/chemse/25.5.493. doi:10.1093/chemse/25.5.493 [DOI] [PubMed] [Google Scholar]
- Page R.E., Erber J., Fondrk M.K. The effect of genotype on response to sucrose and foraging behavior of honeybees (Apis mellifera L.) J. Comp. Phys. A. 1998;182:489–500. doi: 10.1007/s003590050196. doi:10.1007/s003590050196 [DOI] [PubMed] [Google Scholar]
- Pearce J.M. Similarity and discrimination: a selective review and a connectionist model. Psych. Rev. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. doi:10.1037/0033-295X.101.4.587 [DOI] [PubMed] [Google Scholar]
- Petanidou T., Van Laere A., Ellis W.N., Smets E. What shapes amino acid and sugar composition in mediterranean floral nectars? Oikos. 2006;115:155–169. doi:10.1111/j.2006.0030-1299.14487.x [Google Scholar]
- Raguso R.A. Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Curr. Opin. Plant Biol. 2004;7:434–440. doi: 10.1016/j.pbi.2004.05.010. doi:10.1016/j.pbi.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Raguso R.A., Willis M.A. Synergy between visual and olfactory cues in nectar feeding by naïve hawkmoths, Manduca sexta. Anim. Behav. 2002;64:685–695. doi:10.1006/anbe.2002.4010 [Google Scholar]
- Raguso R.A., Willis M.A. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Anim. Behav. 2005;69:407–418. doi:10.1016/j.anbehav.2004.04.015 [Google Scholar]
- Raguso R.A., Levin R.A., Foose S.E., Holmberg M.W., McDade L.A. Fragrance chemistry, nocturnal rhythms, and pollination syndromes in Nicotiana. Phytochem. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. doi:10.1016/S0031-9422(03)00113-4 [DOI] [PubMed] [Google Scholar]
- Rescorla R.A. Stimulus generalization: some predictions from a model of Pavlovian conditioning. J. Exp. Psych. 1976;2:88–96. doi: 10.1037//0097-7403.2.1.88. doi:10.1037/0097-7403.2.1.88 [DOI] [PubMed] [Google Scholar]
- Salzmann C.C., Nardella A.M., Cozzolino S., Schiestl F.P. Variability in floral scent in rewarding and deceptive orchids: the signature of pollinator-imposed selection? Ann. Bot. Lond. 2007;100:757–765. doi: 10.1093/aob/mcm161. doi:10.1093/aob/mcm161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafir S., Reich T., Tsur E., Erev I., Lotem A. Perceptual accuracy and conflicting effects of certainty on risk-taking behaviour. Nature. 2008;453:917–U951. doi: 10.1038/nature06841. doi:10.1038/nature06841 [DOI] [PubMed] [Google Scholar]
- Shepard R.N. Toward a universal law of generalization for psychological science. Science. 1987;237:1317–1323. doi: 10.1126/science.3629243. doi:10.1126/science.3629243 [DOI] [PubMed] [Google Scholar]
- Singaravelan N., Nee'man G., Inbar M., Izhaki I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J. Chem. Ecol. 2005;31:2791–2804. doi: 10.1007/s10886-005-8394-z. doi:10.1007/s10886-005-8394-z [DOI] [PubMed] [Google Scholar]
- Spence K. The differential response in animals to stimuli varying within a single dimension. Psych. Rev. 1937;44:430–444. doi:10.1037/h0062885 [Google Scholar]
- ten Cate C., Rowe C. Biases in signal evolution: learning makes a difference. Trends Ecol. Evol. 2007;22:380–387. doi: 10.1016/j.tree.2007.03.006. doi:10.1016/j.tree.2007.03.006 [DOI] [PubMed] [Google Scholar]
- ten Cate C., Verzijden M.N., Etman E. Sexual imprinting can induce sexual preferences for exaggerated parental traits. Curr. Biol. 2006;16:1128–1132. doi: 10.1016/j.cub.2006.03.068. doi:10.1016/j.cub.2006.03.068 [DOI] [PubMed] [Google Scholar]
- Waddington K.D. Subjective evaluation and choice behavior by nectar- and pollen-collecting bees. In: Chittka L.A., Thomson J.D., editors. Cognitive ecology of pollination. Cambridge University Press; Cambridge, UK: 2001. pp. 41–60. [Google Scholar]
- Waller G.D., Carpenter E.W., Ziehl O.A. Potassium in onion nectar and its probable effect on attractiveness of onion flowers to honey bees. J. Am. Soc. Hortic. Sci. 1972;97:535–539. [Google Scholar]
- Wise P.M., Olsson M.J., Cain W.S. Quantification of odor quality. Chem. Senses. 2000;25:429–443. doi: 10.1093/chemse/25.4.429. doi:10.1093/chemse/25.4.429 [DOI] [PubMed] [Google Scholar]
- Wright G.A., Smith B.H. Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera) Chem. Senses. 2004;29:127–135. doi: 10.1093/chemse/bjh016. doi:10.1093/chemse/bjh016 [DOI] [PubMed] [Google Scholar]
- Wright G.A., Thomson M.G.A. Odor perception and variability in natural odor scenes. In: Romeo J., editor. Chemical ecology and phytochemistry of forest ecosystems. Elsevier; Recent Advances in Phytochemistry. Amsterdam, The Netherlands: 2005. pp. 191–226. [Google Scholar]
- Wright G.A., Thomson M.G.A., Smith B.H. Odour concentration affects odour identity in honeybees. Proc. R. Soc. B. 2005;272:2417–2422. doi: 10.1098/rspb.2005.3252. doi:10.1098/rspb.2005.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G.A., Kottcamp S., Thomson M.G.A. Generalization mediates sensitivity to complex odor features in the honeybee. PLoS ONE. 2008;3:e1704. doi: 10.1371/journal.pone.0001704. doi:10.1371/journal.pone.0001704 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Symmetry of peak shift in an olfactory gradient