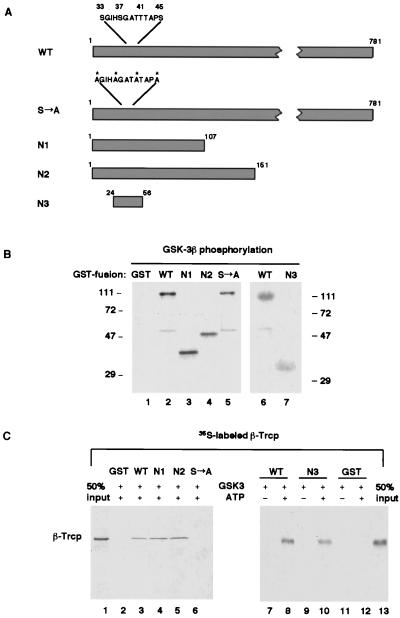
Figure 4.
Phosphorylation of the amino-terminal region of β-catenin is necessary and sufficient for recognition by β-Trcp in vitro. (A) Schematic diagram of the wild-type and mutant derivatives of β-catenin. The four critical serine/threonine residues (S33, S37, T41, and S45), alanine substitutions of these residues in the S→A mutant, and surrounding residues are highlighted. Note that the Arm-repeat region that is required for Axin or TCF binding starts from residue 131. (B) Phosphorylation of β-catenin and its mutant derivatives (as GST-fusion proteins) by purified recombinant GSK-3β. Note that GST was not a substrate for GSK-3β. (C) On phosphorylation, N1, N2, and N3, but not β-catenin (S→A) were effectively recognized by β-Trcp. Lanes 1 and 13 represent 50% input of 35S-labeled β-Trcp in each GST-pull-down assay.