FIG. 2.
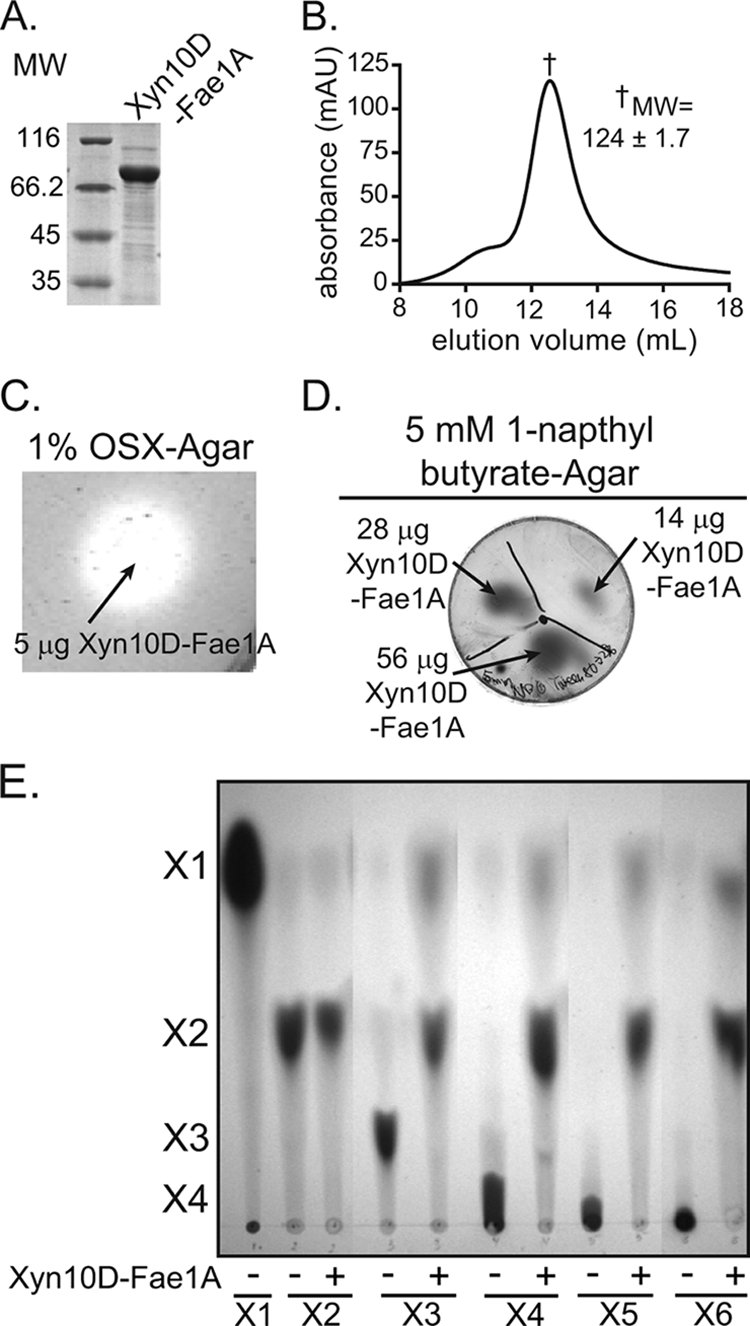
xyn10D-fae1A encodes a bifunctional xylanase-esterase. (A) Purification of recombinant Xyn10D-Fae1A. The eluate from cobalt chelate chromatography was analyzed by 12% SDS-PAGE, followed by Coomassie brilliant blue G-250 staining. MW, molecular weight (in thousands). (B) Gel filtration chromatography. The size of purified Xyn10D-Fae1A was estimated by size exclusion chromatography. The molecular weight of Xyn10D-Fae1A was calculated from the retention time of the peak absorbance by comparison with calibration standards having known molecular weights. Molecular weight is reported as the mean ± standard deviation from three independent experiments. mAU, milli-absorbance units. (C) Depolymerization of OSX. Xyn10D-Fae1A was assessed for its capacity to depolymerize OSX by incubating the protein on an agar plate infused with OSX followed by staining with Congo red. (D) Hydrolysis of 1-napthyl butyrate. Xyn10D-Fae1A was assessed for its capacity to hydrolyze 1-naphtyl butyrate by incubating the protein on an agar plate infused with 1-NB followed by development with fast garnet GBC sulfate. (E) Hydrolysis of xylo-oligosaccharides. Xyn10D-Fae1A-catalyzed hydrolysis of xylo-oligosaccharides (X2 to X6) was assessed by incubating the enzyme with each substrate and then resolving the products by TLC followed by staining with methanolic orcinol.
