Abstract
By utilizing the microdialysis technique, we investigated the pharmacokinetic profile of voriconazole in the interstitium of the lungs and skeletal muscle tissue of rats after a single intravenous dose under healthy and inflammatory conditions. As expected, voriconazole penetrated excellently into the interstitium of tissues, and its levels were descriptively almost identical to free concentration-versus-time profiles in plasma.
The clinical efficacy of voriconazole is thought to be strongly associated with its level of exposure in plasma and the infected site (1). In contrast, the likelihood for the occurrence of severe untoward effects, such as neurotoxicity, has been linked to inappropriate dosing or dosing intervals associated with plasma trough levels of higher than 5.5 mg/liter (2, 8). In addition, the pharmacokinetic profile of voriconazole is nonlinear in all species, making adequate drug dosing a very difficult task in many clinical situations (12). Intrasubject and intersubject variability was shown to range 100-fold depending on age, liver function, genetic polymorphism, dose, concomitant medication, and concurrent illness (7).
In the present study, we explored the pharmacokinetic profile of voriconazole in plasma, the interstitium of lung tissue, and skeletal muscle after administration of a single intravenous dose of 6 mg/kg body weight in rats (Table 1). We also were interested to see whether severe inflammation affects voriconazole's pharmacokinetic profile and expanded the present experimental setting by inducing severe inflammation in a second cohort of rats. These rats received a single intraperitoneal dose of 250 μg of lipopolysaccharide (LPS) and showed symptoms of severe inflammatory response (SIR) within 4 h postadministration.
TABLE 1.
Pharmacokinetic data of voriconazole in plasma (total) and in muscle and lung tissue after a single intravenous dose of 6 mg/kg body weight in healthy rats (n = 7) and in rats presenting with SIR (n = 4)a
| Group | Compartment | AUC0-6h (mg·min/liter) | Cmax (mg/liter) | Tmax (min) | t1/2 (min) |
|---|---|---|---|---|---|
| Healthy | Plasma (total) | 1,179 ± 249 | 5.4 ± 1.6 | 49 ± 32 | 610 ± 334 |
| Lung free | 608 ± 164 | 2.6 ± 0.7 | 137 ± 62 | NA | |
| Muscle free | 530 ± 95 | 2.2 ± 0.4 | 195 ± 91 | NA | |
| Inflamed lungs | Plasma (total) | 1,364 ± 187 | 6.3 ± 1.1 | 15 ± 0 | 677 ± 441 |
| Lung free | 490 ± 159 | 2.3 ± 0.5 | 112 ± 45 | NA | |
| Muscle free | 507 ± 127 | 2.2 ± 0.5 | 130 ± 46 | NA |
Data are expressed as means ± standard deviation. NA, not applicable; AUC0-6h, area under the concentration-time curve from 0 to 6 h; Cmax, maximum concentration of drug in plasma; Tmax, time to Cmax; t1/2, half-life (to be used only with caution as the pharmacokinetic profile is convex after 6 h).
The study protocol was approved by the local Animal Welfare Committee. In total, 22 rats were used in the study. However, only 11 rats (7 were healthy, 4 received LPS) could be used for data analysis as 3 rats were employed for the optimization of study procedures. Eight other rats died during study-related procedures or from complications of SIR. LPS (Escherichia coli serotype 0111:B4; Sigma-Aldrich, Steinheim, Germany) was administered to male Wistar rats (Charles River WIGA GmbH, Sulzfeld, Germany) weighing between 300 and 450 g approximately 9 h before the start of anesthesia and surgical procedures. Death during study procedures was related to therapy-nonresponsive hypotension, edema of the lungs, cardiac ventricular fibrillation, or major bleeding.
The concentrations of voriconazole in the interstitial space fluid (ISF) of skeletal muscle and lung tissue were assessed by utilizing the microdialysis technique. The principle of microdialysis was described previously in detail (5). Voriconazole was quantified by a validated high-performance liquid chromatography method, applying moderate modifications (6). The limit of quantification, accuracy, and precision of the modified method were 0.1 mg/liter, 12.0%, and 5.2% for plasma and 0.2 mg/liter, 7.5%, and 2.7% for microdialysates, respectively.
Plasma protein binding was intensively investigated previously and was reported to be 66% in female and male rats. Free plasma concentrations of voriconazole were calculated from plasma protein binding data obtained from the medical literature (10). Pharmacokinetic calculations were carried out by use of commercially available computer software (Kinetica, version 3.0; InnaPhase, Philadelphia, PA). Voriconazole shows a convex plasma pharmacokinetic profile approximately 6 h after intravenous administration (10) which, in the present study, precludes the use of the elimination rate constant (ke) for the calculation of the last voriconazole concentrations. Thus, the experiment presented here is descriptive only.
From the present data, we obtained circumstantial evidence that voriconazole penetrates excellently into the ISF of soft tissues. Severe inflammation as induced by the administration of high-dose LPS had no descriptive effect on voriconazole's concentration-versus-time profiles in plasma, lung tissue, and skeletal muscle compared to that with healthy conditions (Fig. 1a to c).
FIG. 1.
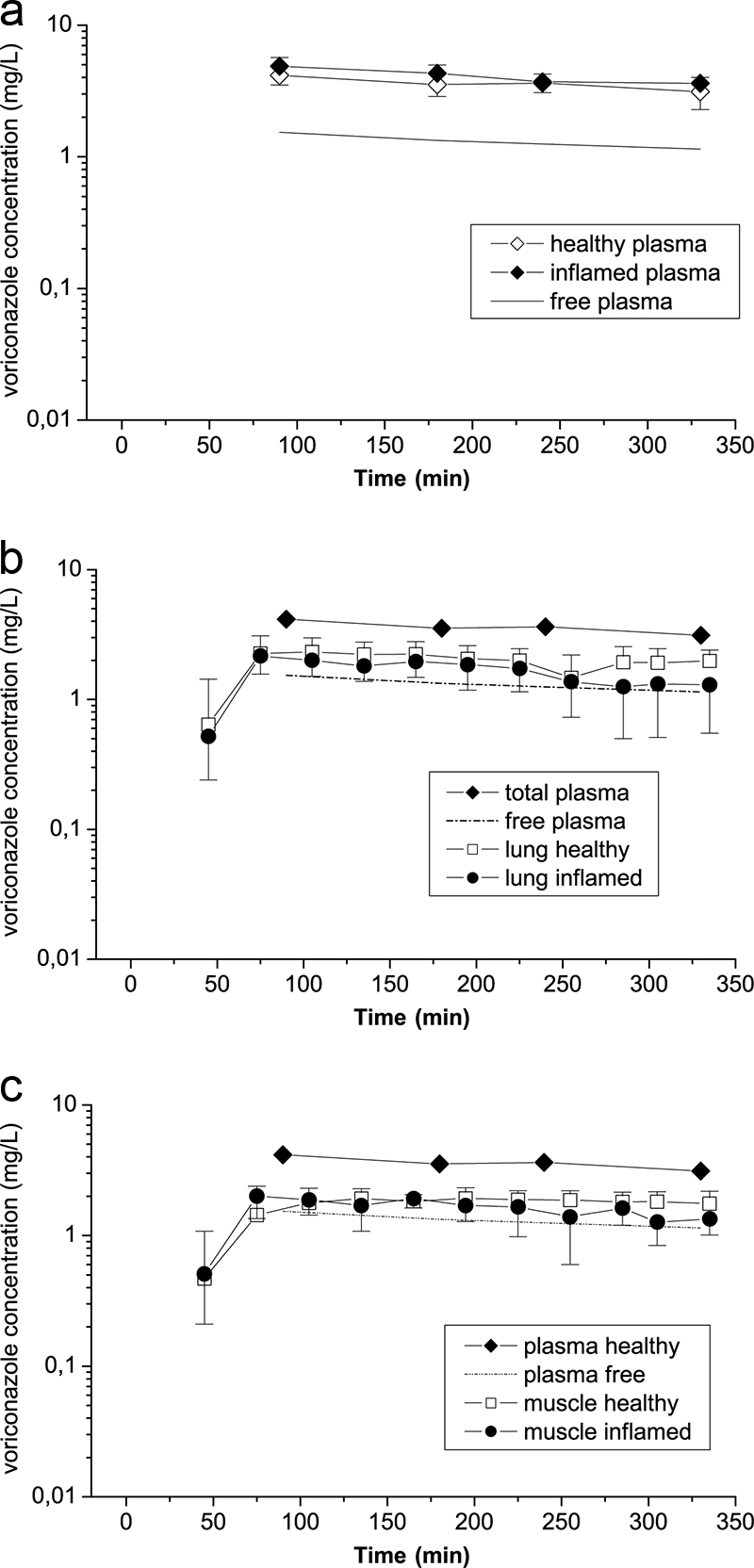
Shown are time-concentration profiles of voriconazole in plasma (a), lungs (b), and skeletal muscle tissue (c) in healthy rats (n = 7) and rats suffering from severe symptoms of inflammatory response after endotoxin challenge with LPS (n = 4). The free concentration-versus-time profile of voriconazole in plasma (estimated from plasma protein binding data from the literature) represents the mean of the inflamed (n = 4) and healthy (n = 7) groups of rats.
The findings of our study are of high clinical relevance as recent studies suggest that concentrations of hydrophilic antiinfectives may be markedly reduced in inflammatory states (4, 11). For children and adults, monitoring of voriconazole plasma concentrations was recently proposed to be advisable to prevent clinical failure due to underdosing or toxicity related to excessive dosing (8, 9, 12).
Effective concentrations of voriconazole at the site of infection are a prerequisite for the successful treatment of serious fungal infections, but no adequate tool is currently available to give an appropriate estimate on voriconazole's concentration at the infected site. Thus, we measured the concentrations of voriconazole in the ISF of lung tissue and skeletal muscle tissue under inflamed and healthy conditions and compared these data to corresponding free plasma levels, an approach which offers several advantages. First, blood can be withdrawn easily during daily routine clinical measures, and second, the skeletal muscle compartment has been suggested to serve as an appropriate surrogate tissue for the estimation of corresponding concentrations of antiinfectives in the ISF of lungs (3), particularly in cases in which microdialysis probe insertion into the lung is not feasible (13). In addition, an increasing number of hospitals are about to offer sensitive and validated methods for the measurement of voriconazole concentrations in bodily fluids.
As free concentrations of voriconazole in plasma were almost identical to free concentration-versus-time profiles in lung and skeletal muscle tissue over the entire observation period of 6 h, it is tempting to hypothesize that the knowledge of the free fraction in plasma may serve as a good estimate of voriconazole concentration in the ISF in lung tissue and skeletal muscle even under inflammatory conditions (Fig. 1b and c). Hence, based on single-dose measurements, we offer a rough estimation of voriconazole concentrations in the interstitium of relevant target sites. We provide evidence that free voriconazole in plasma equilibrates completely with the extracellular space fluid of lung tissue and skeletal muscle under inflammatory and healthy conditions (Fig. 1a to c). However, it is noteworthy to mention that this is a comparison between observed (tissue) and estimated (free plasma) voriconazole concentrations and that drug protein binding in plasma may be affected by LPS-induced severe septic shock. Finally, our data were derived from a well-performed preclinical experimental setting with rats and thus need to be verified in a prospective, controlled clinical trial with human subjects. The major metabolite of voriconazole, N-oxide (UK-121,265), was not measured in the present study as it is not considered to contribute to the antifungal efficacy of voriconazole (10).
In summary, based on the concept that the efficacy of voriconazole is closely related to drug exposure at the infected site, we conclude that the measurement of voriconazole concentrations in plasma could help to reduce untoward side effects due to toxic plasma levels and in parallel may increase success rates in underdosed patients. Currently available pharmacokinetic-pharmacodynamic indices and breakpoints for voriconazole are not yet firmly established, but with some limitations they may be considered predictive of clinical success and safety. Their implementation in the strategy and decision making on appropriate dosing regimens may be advisable.
Acknowledgments
This work was supported by an unrestricted grant from the Pfizer Corporation, Vienna, Austria.
We are indebted to Frieda Sodeck and others who contributed to the success of the present study.
C.J. and K.H.K. are consultants for pharmaceutical companies. C.J. is managing director of J&P Medical Research Ltd., Vienna, Austria, and visiting Associate Professor at Harvard Medical School, Boston, MA. All other authors declare having no relationship with companies that make products relevant to the manuscript and having no conflicts of interest with the present work.
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, A. E., S. Modi, S. J. Howard, C. B. Moore, B. G. Keevil, and D. W. Denning. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. [DOI] [PubMed] [Google Scholar]
- 3.Dahyot, C., S. Marchand, G. L. Pessini, C. Pariat, B. Debaene, W. Couet, and O. Mimoz. 2006. Microdialysis study of imipenem distribution in skeletal muscle and lung extracellular fluids of Acinetobacter baumannii-infected rats. Antimicrob. Agents Chemother. 50:2265-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joukhadar, C., M. Frossard, B. X. Mayer, M. Brunner, N. Klein, P. Siostrzonek, H. G. Eichler, and M. Müller. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385-391. [DOI] [PubMed] [Google Scholar]
- 5.Joukhadar, C., and M. Müller. 2005. Microdialysis: current applications in clinical pharmacokinetic studies and its potential role in the future. Clin. Pharmacokinet. 44:895-913. [DOI] [PubMed] [Google Scholar]
- 6.Khoschsorur, G., F. Fruehwirt, and S. Zelter. 2005. Isocratic high-performance liquid chromatographic method with ultraviolet detection for simultaneous determination of levels of voriconazole and itraconazole and its hydroxyl metabolite in human serum. Antimicrob. Agents Chemother. 49:3569-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis, R. E. 2008. What is the therapeutic range for voriconazole? Clin. Infect. Dis. 46:212-214. [DOI] [PubMed] [Google Scholar]
- 8.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto, A. C., M. Shah, R. Wynn, and D. W. Denning. 2008. Voriconazole plasma monitoring. Arch. Dis. Child. 93:587-593. [DOI] [PubMed] [Google Scholar]
- 10.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 11.Tegeder, I., A. Schmidtko, L. Bräutigam, A. Kirschbaum, G. Geisslinger, and J. Lötsch. 2002. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 71:325-333. [DOI] [PubMed] [Google Scholar]
- 12.Theuretzbacher, U., F. Ihle, and H. Derendorf. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649-663. [DOI] [PubMed] [Google Scholar]
- 13.Zeitlinger, M., M. Müller, and C. Joukhadar. 2005. Lung microdialysis—a powerful tool for the determination of exogenous and endogenous compounds in the lower respiratory tract (mini-review). AAPS J. 7:E600-E608. [DOI] [PMC free article] [PubMed] [Google Scholar]


