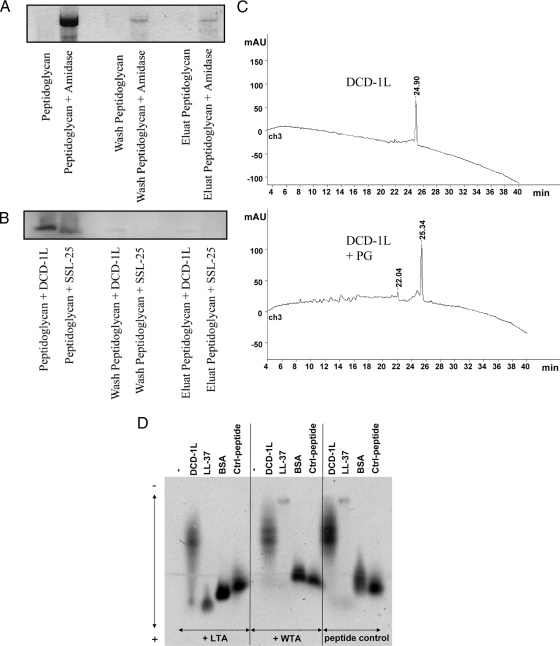
FIG. 4.
DCD-derived peptides show only weak binding to PG and teichoic acids of gram-positive bacteria. (A to C) Shown is a PG binding assay. Peptides and amidase as a control were incubated with PG. After incubation, supernatant, wash fraction, and eluate were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie blue. (A) S. aureus amidase can be detected in all fractions (supernatant, wash, and elution), indicating binding to PG. (B) DCD-1L and SSL-25 could be detected only in supernatant; however, there was no visible detection in the wash or the elution fraction. (C) PG binding was observed via high-pressure liquid chromatography by comparing decreases of DCD-1L before and after incubation with PG. The absence of a decrease in DCD-1L reveals that there was no binding to PG. mAU, milliabsorption units over time (in min). (D) Peptide gel shift assay. FITC-labeled peptides (0.25 mg/ml) were incubated with or without the putative binding partners WTA and LTA (0.75 mg/ml). Native agarose gel electrophoresis of the peptides revealed a measurable gel shift toward the anode due to the negative charge of LTA/WTA when there was binding. Whereas no binding was seen with WTA, DCD-1L-FITC (moderate binding) and LL-37-FITC (strong binding) showed moderate and strong binding, respectively, to LTA. BSA-FITC and another control peptide served as controls. Using lower DCD peptide concentrations did not result in different binding behavior.