Abstract
The proton pump inhibitor omeprazole reduced the intracellular replication of Salmonella enterica serovar Typhimurium in RAW264.7 cells without affecting bacterial growth in vitro or the viability of the host cells. The mechanism was bacteriostatic and interfered with replication mediated by the virulence-associated SPI2 type III secretion system. The proton pump inhibitor bafilomycin A1, in contrast, mediated killing of intracellular bacteria and imposed a marked cytotoxicity on RAW264.7 cells. The two compounds also differentially affected the proinflammatory responses of the infected cells. Bafilomycin A1 enhanced nitric oxide production, whereas omeprazole delayed IκB degradation and blocked nitric oxide production and the secretion of proinflammatory cytokines. These results imply that omeprazole can be used to block the virulence factor-mediated intracellular replication of S. Typhimurium, and that its mechanism of growth inhibition is different from that mediated by bafilomycin A1.
Small molecular organic compounds recently have been used for interfering with bacterial pathogenicity on an experimental basis. Selected salicylidene acylhydrazides, for example, have been used to block the bacterial type III secretion system (T3SS)-dependent intracellular replication of Chlamydia (30, 44) and Salmonella enterica (31) in cultured cells and the T3SS-dependent cytotoxicity of Yersinia pseudotuberculosis (21, 22, 32). Other sets of compounds have been used to block the transcription of virulence factor genes in enteropathogenic Escherichia coli (14) and Vibrio cholerae (20). Such substances thus prevent the expression or functionality of virulence factors without affecting bacterial viability and have the potential to be used as compounds for new antimicrobials as well as tools for dissecting virulence mechanisms (19, 22).
Vacuolar acidification is a normal process of endosomal vesicles and is generated chiefly through the activity of vacuolar H+-ATPases that lodge in the maturing endosome (39). Many pathogens that target the endosomal compartment of host cells rely on vacuolar acidification for their ability to cause disease. For example, decreased vacuolar pH induces the activation of selected bacterial toxins such as cholera and tetanus toxins (27, 43) and the intracellular replication of Leishmania (28) and Salmonella enterica (35). A chemical commonly used for inhibiting vacuolar acidification is the macrolide bafilomycin A1. It acts as a potent irreversible inhibitor of H+-ATPases (40) and interferes with the activity of cholera toxin in liver cells and tetanus toxin in neuronal cells (27, 43). Bafilomycin A1 also has been reported to decrease the intracellular replication of L. monocytogenes and S. enterica serovar Typhimurium in both epithelial and monocytic cells (7, 9, 35).
The ability of S. Typhimurium to cause systemic infection in mice relies on two separate T3SSs (15). These T3SSs are coded for by separate genetic continuums, called Salmonella pathogenicity islands 1 and 2 (SPI1 and SPI2). Effector proteins translocated the SPI1 T3SS mediate bacterial uptake from the intestine (13, 15), whereas SPI2 effectors are needed for subsequent intravacuolar replication in phagocytic cells (6, 15, 23). The induction of SPI2 genes and the assembly of the SPI2 T3SS both require a decrease in pH and phosphate (1, 25), after which selected SPI2 effector proteins interfere with subsequent vacuolar maturation (2, 26).
In this study, we asked whether pharmaceutical proton pump inhibitors could be used as small molecular compounds for a targeted interference with virulence. Thus, we probed for the potential of the H+-ATPase inhibitor omeprazole, a benzimidazole compound that is used for treating gastric ulcer disease and gastroesophagal reflux, to inhibit the proliferation of S. Typhimurium in RAW264.7 cells.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation.
S. enterica serovar Typhimurium strain 14028 (American Type Culture Collection, Manassas, VA) was used throughout the study. For the plasmid segregation experiments, S. Typhimurium 14028 was transformed with plasmid pPir, which codes for ampicillin resistance on a temperature-sensitive replicon (34). SPI2 mutant strains ssaV::Cmr, sifA::Kmr, sseG::Kmr, and sseF::Kmr (1, 2) all were made in the 14028 strain background and were a kind gift from David W. Holden and Jay C. D. Hinton. For microscopy, we used the gfp+ insertion from strain JH3016 (SL1344, rpsM′-gfp+, Cmr) (16) transduced into the 14028 background. Bacteria were grown in Luria-Bertani (LB) medium or on LB agar plates supplemented, when needed, with ampicillin (100 μg/ml; Sigma, St. Louis, MO), chloramphenicol (10 μg/ml; Sigma), or kanamycin sulfate (50 μg/ml; Sigma).
Proton pump inhibitors.
The proton pump inhibitors omeprazole and bafilomycin A1 were solubilized in dimethyl sulfoxide (DMSO). All products were purchased from Sigma. An equivalent amount of DMSO was used as the negative control in all experiments.
In vitro cell culture infection.
The murine macrophage-like cell line RAW264.7 (TIB-71; ATCC) was propagated in RPMI 1640 (Gibco, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (Gibco), l-glutamine (10 mM), HEPES (10 mM), and gentamicin (10 μg/ml) as described by Bjur et al. (4). l-glutamine, HEPES, and gentamicin were purchased from Sigma.
For the infection of nonactivated RAW264.7 cells, bacteria were grown to be noninvasive (4) and opsonized with 10% preimmune BALB/c mouse serum for 30 min at 37°C and subsequently used at a multiplicity of infection of 10. One hour postinfection, cell culture medium containing gentamicin at a concentration of 50 μg/ml was applied, and cells were incubated for 45 min to kill extracellular bacteria. For continued incubations, the killing medium was replaced by maintenance medium containing 10 μg/ml of gentamicin.
At indicated time points postinfection, the cells were lysed by hypotonic lysis (11), and the amount of intracellular bacteria was determined by CFU counts of viable bacteria.
To probe for the effect of proton pump inhibitors, the compounds were added at the indicated concentrations. If not otherwise stated, omeprazole was added to all media and kept for the duration of the experiment. In the case of bafilomycin A1, the cells were treated with the compound 30 min prior to infection, and then fresh medium without bafilomycin A1 was added (35). When testing for cytotoxicity, experiments also were conducted in cell culture medium containing bafilomycin A1 for 16 h. For testing the effect of proton pump inhibitors on nonreplicating bacteria, tetracycline (10 μg/ml; Sigma) was added as reported by Negrea et al. (31).
Determination of NO production.
The production of nitric oxide (NO) was measured through the accumulation of nitrite in the cell culture medium. Nitrite was assayed with the Griess reagent (Merck, Darmstadt, Germany), and the optical densities at 525 nm were converted to micromolar concentrations using defined standards of sodium nitrite (Merck).
Analysis of cell viability.
Cell death was detected using an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Pharmingen) and subsequent analysis by flow cytometry. The procedure also included staining with propidium iodide (PI). The release of lactate dehydrogenase (LDH) was assayed chromogenically using a cytotoxicity detection kit (Roche) according to the manufacturer's instructions.
Immunoblotting.
The degradation of IκB-α and the expression of inducible nitric oxide synthase (iNOS) were assayed by immunoblotting of RAW264.7 cells as described by Eriksson et al. (10). Briefly, adherent cells were washed in phosphate-buffered saline (PBS) and lysed immediately in 100 mM Tris (pH 8) containing 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, and 1% NP-40. All of these chemicals were purchased from Sigma except for NP-40, which was from Merck. Proteins were separated on sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gels (24) and transferred onto HybondP polyvinyl difluoride membranes (Amersham Pharmacia Biotech) as recommended by the manufacturer. IκB-α and iNOS were detected using a rabbit monoclonal anti-IκB-α antibody (Cell Signaling Technology, Danvers, MA) and a rabbit polyclonal anti-iNOS serum (Santa Cruz Biotechnology, Santa Cruz, CA). To confirm the equal loading of the samples, β-actin was detected by immunoblotting in parallel as an internal standard, using a rabbit anti-actin antibody from Sigma. Bound primary antibodies were detected using peroxidase-conjugated anti-rabbit antibodies and the chemiluminescent detection kit SuperSignal from Pierce Biotechnology (Rockford, IL). The PageRuler prestained protein ladder plus (Fermentas, Ontario, Canada) was used as a molecular mass marker.
Measurement of cytokine release.
OptEIA enzyme-linked immunosorbent assay kits (BD Bioscience, Franklin Lakes, NJ) were used to determine the levels of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) in cell culture medium 16 h postinfection. The assays were conducted as defined by the manufacturer.
Fluorescence microscopy.
RAW264.7 cells were grown on coverslips and infected as described above with a green fluorescent protein (GFP)-expressing 14028 S. Typhimurium strain carrying gfp under a constant promoter (16). At 16 h postinfection, the cells were fixed in phosphate-buffered 4% formaldehyde (pH 7.2) for 10 min, washed with PBS, mounted on microscopy slides, and observed using a fluorescence microscope. For each sample, 20 random microscopy fields were selected and processed for statistical analyses. To assess vacuolar acidification, RAW264.7 cells were stained with acridine orange (Sigma) according to Steele-Mortimer et al. (38).
Determination of MICs.
MICs were determined on 96-well microtiter plates by applying twofold dilutions of gentamicin and omeprazole. Assays were conducted in either LB or RPMI-based HEPES-buffered cell culture medium and initiated at a bacterial concentration of 105 CFU per ml.
Statistical analyses.
Each experiment was performed in triplicate and repeated at least two times. A two-sided student's t test was used to determine statistical significance between the values for the different groups.
RESULTS
Omeprazole prevents intracellular replication of S. Typhimurium in a bacteriostatic manner.
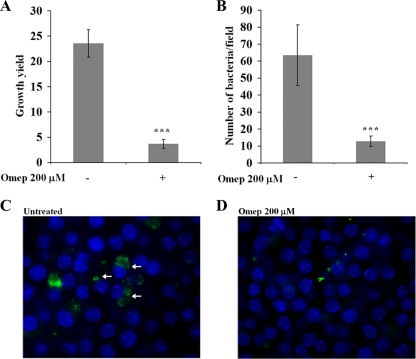
A gentamicin protection assay (37) was used to analyze the impact of omeprazole on the intracellular replication of S. Typhimurium strain 14028 in murine macrophage-like RAW264.7 cells. In untreated cells, we constantly noted a significant increase in intracellular bacteria between 2 and 16 h postinfection (Fig. 1A); however, when the experiment was performed in the presence of omeprazole (200 μM) (8), a significant decrease in the bacterial growth yield was observed (Fig. 1A).
FIG. 1.
Influence of omeprazole (Omep) on the growth yield of S. Typhimurium 14028 bacteria in RAW264.7 cells. The increase in intracellular bacteria between 2 and 16 h postinfection was determined by viable counting, and the ratio is displayed as the growth yield (A) or by enumerating the mean number of intracellular bacteria per microscopic field at 16 h postinfection (B). Representative microscopic fields for untreated (C) and omeprazole-treated (D) infected cells are shown. Nuclei of RAW264.7 cells have been stained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue), and the bacteria are revealed as green through the expression of GFP. For each sample, 20 random microscopy fields were selected and processed for statistic analyses. The arrows indicate the perinuclear localization of bacteria. The statistical difference between the growth yields was confirmed in a paired student's t test (***, P < 0.0005).
We next conducted microscopy using a GFP-expressing strain of S. Typhimurium 14028. Infected RAW264.7 cells were fixed 16 h postinfection and probed for GFP-expressing bacteria. In agreement with the results observed using viable counts (Fig. 1A), omeprazole-treated cells contained less intracellular bacteria (Fig. 1D) than untreated cells (Fig. 1C). A statistical analysis of the number of fluorescent intracellular bacteria per microscopy field confirmed that omeprazole inhibited bacterial intracellular growth (Fig. 1B). In infected untreated controls, microscopy revealed a pattern typical of infected cultured cells: cells containing some S. Typhimurium bacteria and cells with multiple bacteria clustered in a perinuclear region (Fig. 1C).
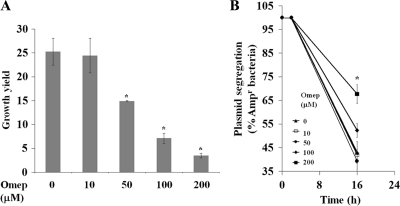
To further analyze the effect of omeprazole on the intracellular growth of S. Typhimurium, RAW264.7 cells were infected with a variant of S. Typhimurium 14028 that carried a plasmid that codes for ampicillin resistance on a temperature-sensitive replicon (3, 34). As this strain divides at 37°C, the plasmid segregates and allows the detection of actual replication (3). In these experiments, omeprazole affected bacterial intracellular net growth and plasmid segregation at concentrations as low as 50 μM and substantially decreased the bacterial intracellular growth and plasmid segregation at 200 μM (Fig. 2A and B). Taken together, these results imply that omeprazole exerts a bacteriostatic mechanism of growth inhibition.
FIG. 2.
Omeprazole (Omep) shows a concentration-dependent inhibition of intracellular replication of S. Typhimurium 14028 in RAW264.7 cells. (A) Samples of intracellular bacteria were plated 2 and 16 h postinfection with RAW264.7 cells treated with a DMSO control or with different concentrations of omeprazole. The growth yield reflects the ratio of the viable counts for the two time points. (B) The concomitant segregation rate for a plasmid-carried ampicillin resistance marker for S. Typhimurium 14028 residing in RAW264.7 cells is consistent with a bacteriostatic effect of omeprazole on bacterial intracellular growth. The statistical difference between the growth yields was confirmed in a paired student's t test (*, P < 0.05).
Omeprazole and bafilomycin A1 differently inhibit intracellular replication of S. Typhimurium in RAW264.7 cells.
We next analyzed the abilities of the proton pump inhibitors omeprazole and bafilomycin A1 to affect the growth of S. Typhimurium in vitro or in murine macrophage-like RAW264.7 cells.
Neither proton pump inhibitor (omeprazole at 50 to 200 μM and bafilomycin A1 at 100 nM) affected bacterial replication in Luria broth or in cell culture medium, nor did the compounds alter the gentamicin MIC for S. Typhimurium 14028 in these media (data not shown). Thus, neither omeprazole nor bafilomycin A1 inhibited bacterial in vitro replication, nor did the compounds sensitize bacteria to gentamicin at relevant experimental concentrations of proton pump inhibitors.
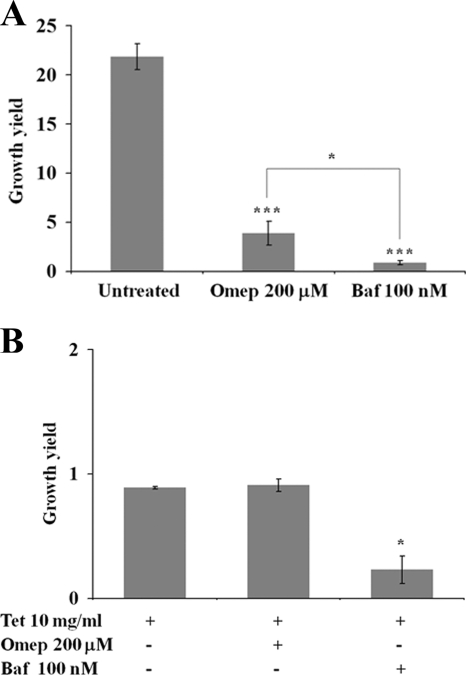
In accordance with the results presented above (Fig. 1), we noted a two- to fourfold net increase in intracellular bacteria when using omeprazole at 200 μM 16 h postinfection (Fig. 3A). Bafilomycin A1 at 100 nM completely prevented net bacterial replication from 2 to 16 h of incubation, even when the compound was applied only for 30 min prior to infection (Fig. 3A).
FIG. 3.
Proton pump inhibitors differently affect the viability of intracellular S. Typhimurium. (A) Bafilomycin A1 had a stronger inhibitory effect on the intracellular replication of S. Typhimurium in RAW264.7 cells. (B) RAW264.7 cells infected with S. Typhimurium 14028 were treated simultaneously with tetracycline (10 μg/ml) to cause a reversible inhibition of bacterial protein synthesis and replication, in conjunction with either omeprazole (Omep) or bafilomycin A1 (Baf). This analysis showed that tetracycline completely inhibited bacterial intracellular replication, and that there was no additive effect by combining omeprazole and tetracycline (Tet). In contrast, bafilomycin A1 caused a significant decrease in the numbers of tetracycline-treated bacteria. The statistical difference between the growth yields was confirmed in a paired student's t test (*, P < 0.05).
The application of tetracycline at 10 μg/ml causes a reversible blockage in the intracellular replication of S. Typhimurium in RAW264.7 cells (31). To test whether the two proton pump inhibitors affected nonreplicating bacteria, we infected RAW264.7 cells with S. Typhimurium strain 14028 and included tetracycline in the presence or absence of the proton pump inhibitors. At 16 h postinfection in the presence of tetracycline, we did not detect growth or a decrease in bacterial numbers. The yield of intracellular bacteria remained unaltered when using both tetracycline and omeprazole (Fig. 3B). Thus, omeprazole did not further affect the viability of nonreplicating intracellular bacteria.
The application of bafilomycin A1 to tetracycline-treated RAW264.7 cells resulted in a significant decrease in the numbers of bacteria recovered 16 h postinfection (Fig. 3B). These results suggest that bafilomycin A1, in contrast to omeprazole, mediated the killing of nonreplicating intracellular bacteria.
Omeprazole acts before initiation of bacterial intracellular replication.
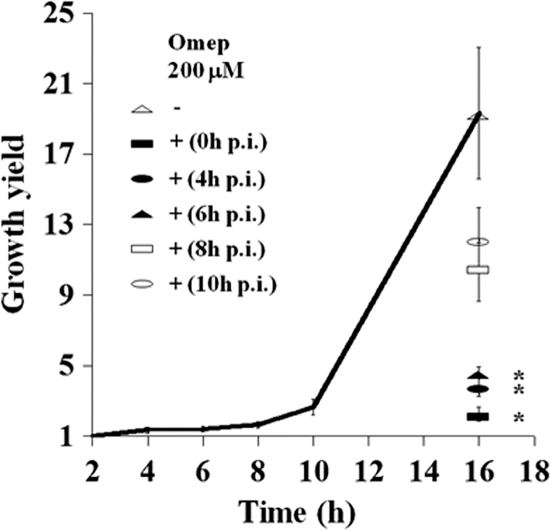
We analyzed the kinetics of intracellular growth in the gentamicin protection assay by determining the intracellular growth yield at 2, 4, 6, 8, and 10 h postinfection. These experiments revealed that net growth became detectable at 8 h postinfection (Fig. 4). The application of omeprazole (200 μM) at 4 or 6 h postinfection inhibited net growth as measured 16 h postinfection, whereas growth was significantly less affected when omeprazole was applied at 8 or 10 h postinfection (Fig. 4). The application of omeprazole for 3 h before infection resulted in a substantial inhibition of bacterial intracellular replication, even though the compound was omitted at later stages of the infection (data not shown).
FIG. 4.
Growth inhibition of intracellular S. Typhimurium relies on omeprazole (Omep) application at early stages of infection. S. Typhimurium 14028 begins to show intracellular replication in RAW264.7 cells at 8 h postinfection. The application of omeprazole before this time point results in the substantial inhibition of the intracellular growth yield as measured at 16 h postinfection. The statistical difference between the growth yields was confirmed in a paired student's t test (*, P < 0.05).
These observations implied that omeprazole efficiently prevented bacterial intracellular replication, but it did so only if applied before intracellular replication was initiated. This suggested that omeprazole inhibits the expression of bacterial virulence factors that promote growth. After such factors have been expressed, omeprazole would lose its antiproliferating effect. Based on the literature, one such virulence determinant could be the SPI2 T3SS in terms of induction kinetics (11).
Omeprazole affects SPI2-mediated intracellular replication.
The complete blockage of the SPI2 T3SS through the mutational inactivation of the SPI2 T3SS machinery strongly reduces the ability of S. Typhimurium 14028 to replicate in RAW264.7 cells. Likewise, selected SPI2 effector proteins encoded by SPI2 and SPI2-associated genes, such as sifA, are needed equally for the efficient intracellular replication of S. Typhimurium in RAW264.7 cells (1, 12).
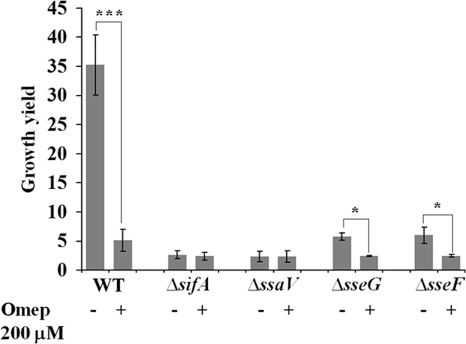
In accordance with previous publications (1, 12), we observed a significantly decreased intracellular growth yield in RAW264.7 cells for sifA, sseG, and sseF effector mutants and for the ssaV mutant which is defective in the SPI2 T3SS apparatus (Fig. 5). However, both of the sifA and ssaV mutants, the mutants with the most reduced growth yield, repeatedly generated a two- to threefold increase in net growth in relation to the amount of bacteria that were phagocytosed initially (Fig. 5). These observations showed that certain SPI2 genes are needed for bacterial intracellular replication, as expected. However, consistently with other publications (1-3, 12), a measurable intracellular replication could be observed even in the absence of SPI2 activity (Fig. 3B and 5).
FIG. 5.
Intracellular replication of selected SPI2 mutants of S. Typhimurium 14028 in RAW264.7 cells reveals a residual SPI2-independent replication that is not affected by omeprazole (Omep). The increase in intracellular bacteria between 2 and 16 h postinfection in the absence or presence of omeprazole (at 200 μM) was determined by viable counting, and the ratio is displayed as the growth yield. The statistical difference between the growth yields was confirmed in a paired student's t test (*, P < 0.05; ***, P < 0.0005). WT, wild type.
As the effect of omeprazole on the intracellular replication of S. Typhimurium 14028 mimicked that of the sifA and ssaV mutations, we next asked whether omeprazole would act through interference with SPI2. If so, then no additive growth inhibition would be expected for the sifA or ssaV mutant upon use of omeprazole. However, if omeprazole acted via a different pathway, then the addition of omeprazole would pose an additive inhibitory effect on the residual growth of the sifA and ssaV mutants. Thus, we determined the intracellular growth yields in RAW264.7 cells of the wild-type and mutant strains of S. Typhimurium in the presence and absence of omeprazole.
When using omeprazole at 200 μM, the growth yield of the sseG and sseF mutants was reduced twofold, whereas the growth of the sifA and ssaV mutants was unaffected (Fig. 5). Significantly, in omeprazole-treated cells the growth yield of the wild-type and mutant strains reached about the same level of decreased replication. This indicated that there exists major SPI2-dependent and minor SPI2-independent growth of S. Typhimurium in RAW264.7 cells and that omeprazole affects only the SPI2-mediated growth.
Omeprazole does not affect vacuolar acidification in RAW264.7 cells.
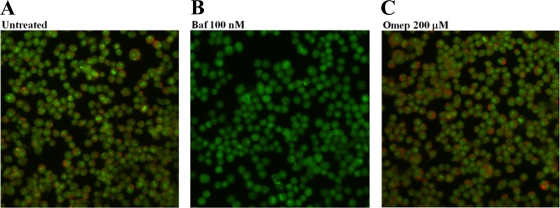
We continued by asking whether omeprazole at the concentration used in this study affected vacuolar pH. For this, we applied staining with acridine orange, a dye that shifts fluorescence emission upon protonation. In accordance with Steele-Mortimer et al. (42), acridine orange-stained RAW264.7 cells revealed intravacuolar orange staining that is indicative of acidified vacuoles (Fig. 6A), whereas treatment with bafilomycin A1 resulted in a total absence of such staining (Fig. 6B). Omeprazole-treated acridine orange-stained RAW264.7 cells still revealed acidified vacuoles (Fig. 6C).
FIG. 6.
Acridine orange staining of RAW264.7 cells. Untreated (A) and omeprazole (Omep)-treated (C) cells show an acidified vacuolar staining that is absent in cells treated with bafilomycin A1 (Baf) (B).
Omeprazole and bafilomycin A1 differentially affect inflammatory activation of infected RAW264.7 cells.
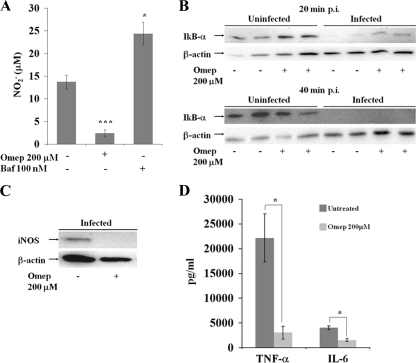
Proton pump inhibitors have been reported to affect inflammatory signaling (17) and the expression of inducible nitric oxide synthase (iNOS) in macrophages and macrophage-like cells (18). The expression of iNOS also can be induced in murine monocytic cells through infection with S. Typhimurium, whereupon subsequent NO production inhibits the intracellular replication of S. Typhimurium (4, 42). As the immunomodulatory propensities of proton pump inhibitors had not been harmonized previously to the outcome of infection assays, we set out to probe whether omeprazole and bafilomycin A1 affected the ability of infected RAW264.7 cells to produce NO in response to intracellular S. Typhimurium. These experiments showed that omeprazole, surprisingly, efficiently abrogated the ability of infected RAW264.7 cells to produce NO, whereas bafilomycin A1 increased the NO production in the infected cells (Fig. 7A).
FIG. 7.
(A) Nitric oxide levels in infected untreated control cells and omeprazole (Omep)-treated or bafilomycin A1 (Baf)-treated cells. Immunoblot signals of infected RAW264.7 cells for IκB-α at 20 or 40 min postinfection (p.i.) (B) or iNOS at 16 h postinfection (C) in the presence or absence of omeprazole are shown. β-Actin expression served as a loading control. (D) Levels of the secreted cytokines TNF-α and IL-6 in the supernatants 16 h postinfection in the untreated or omeprazole-treated samples. The statistical differences between NO and cytokine secretion were confirmed in a paired student's t test (*, P < 0.05; ***, P < 0.0005).
The downregulation of NO production in infected omeprazole-treated cells was associated with the inhibition of iNOS expression (Fig. 7C), low secreted levels of the cytokines IL-6 and TNF-α (Fig. 7D), and a delayed degradation of IκB-α (Fig. 7B) in response to infection. Thus, the two proton pump inhibitors differentially affected infected RAW264.7 cells, with omeprazole blocking inflammatory signaling and the expression of iNOS.
Proton pump inhibitors and viability of RAW264.7 cells.
The gentamicin protection assay relies on the fact that the intact eukaryotic cell protects intracellular bacteria from external aminoglucosides (37). Vacuolar proton pump inhibitors have been reported to induce apoptosis in cultured mammalian cells (5, 29, 45). Host cell death, in turn, would expose intracellular bacteria to gentamicin, which would result in the killing of the bacteria in the cell culture setting. As this aspect had not been included in previous studies connecting bafilomycin A1 to bacterial virulence factor activity, we analyzed omeprazole and bafilomycin A1 for their effects on the viability of macrophage-like RAW264.7 cells.
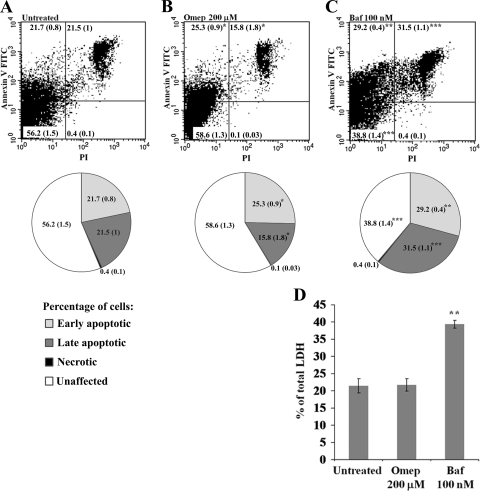
RAW264.7 cells were grown in the absence or in the presence of either omeprazole or bafilomycin A1 for 16 h. Thereafter, apoptosis and cell death was recorded by staining with annexin V-FITC and PI and through the measurement of the release of LDH into the cell culture medium.
After 16 h of exposure to omeprazole, there were no substantial differences in apoptotic responses compared to those of untreated control cells (Fig. 8A, B, and D). Furthermore, it was possible to culture RAW264.7 cells in the presence of omeprazole at 200 μM without affecting cell viability (data not shown). In contrast, bafilomycin A1 induced a significant increase in the number of annexin V-FITC- and PI-positive cells and an increase in the LDH release in RAW264.7 cells (Fig. 8A, C, and D). A large population of cells stained positive for both annexin V-FITC and PI, suggesting that a great number of bafilomycin A1-treated cells were in a late apoptotic phase 16 h after exposure to the compound. Moreover, at 16 h of cultivation, a substantial increase in staining for annexin V and PI was recorded for cells that were exposed to bafilomycin A1 for only 30 min (data not shown). Thus, compared to bafilomycin A1, omeprazole appeared significantly less cytotoxic at the concentrations reported in this study.
FIG. 8.
Annexin V-FITC and PI staining of RAW264.7 cells treated with DMSO (untreated control) (A) or exposed to omeprazole (Omep) (B) or bafilomycin A1 (Baf) (C) for 16 h, as analyzed by fluorescent-activated cell sorting. The numbers are given as percentages of all cells counted, with the standard deviations from the means in brackets. (D) Corresponding LDH levels released into the cell culture medium at 16 h postinfection. The statistical differences were confirmed in a paired student's t test (*, P < 0.05; **, P < 0.001; ***, P < 0.0005).
DISCUSSION
The expression of bacterial virulence factors is highly regulated (36), and conditions defined by low pH and low concentrations of magnesium and phosphate are environmental cues that, in S. Typhimurium, activate the expression and functionality of the virulence-associated SPI2 T3SS (1, 25). Here, we have probed for the potential to interfere with the intravacuolar low pH that acts as a signal for SPI2 T3SS activation in S. Typhimurium through the use of two well-defined vacuolar proton pump inhibitors. Our results showed that omeprazole imposed a bacteriostatic effect on intracellular S. Typhimurium, and that only the SPI2-dependent replication was affected when the bacteria infected murine macrophage-like RAW264.7 cells. We also could show that omeprazole became less effective if applied at later stages of the infection, when the expression of SPI2 genes already has occurred and replication already has started (11). These results would be consistent with omeprazole affecting the expression or activity of SPI2. However, under the experimental settings applied, omeprazole treatment did not abrogate the intravacuolar pH in RAW264.7 cells.
The literature defines various effects of the proton pump inhibitor bafilomycin A1 on the intracellular replication of S. Typhimurium. An early report noted a drastic antibacterial effect of bafilomycin A1 on S. Typhimurium replicating in RAW264.7 cells (35). This effect appeared bactericidal, even when the compound was applied at later stages of the intracellular infection. However, in a subsequent report bafilomycin A1 failed to prevent the intracellular replication of S. Typhimurium in RAW264.7 cells (38). While we also observed a potent antibacterial effect of bafilomycin A1 on intracellular S. Typhimurium, its effect appeared to be different from that of omeprazole. Bafilomycin A1 acted on all intracellular bacteria through a bactericidal mechanism, while omeprazole affected only the virulence factor-mediated replication of bacteria and had no effect on nonreplicating bacteria.
Murine monocytic cells respond to salmonella infection by producing iNOS and, subsequently, NO, which suppress the net intracellular replication of S. Typhimurium (4, 6, 10, 42). One explanation for the different mechanisms of the antimicrobial action of bafilomycin A1 and omeprazole relates to the different abilities of the two proton pump inhibitors to evoke an NO response in infected cells. Whereas bafilomycin A1 enhanced host cell antibacterial potential and NO production, omeprazole surprisingly simultaneously blocked the ability of the infected RAW264.7 cells to mount an NO response and bacterial intracellular replication. Omeprazole-treated infected RAW264.7 cells also were impaired in the secretion of TNF-α and IL-6 and in iNOS expression, and they showed a delay in IκB degradation, implying that omeprazole suppressed inflammatory signaling. That omeprazole blocked SPI2-assisted replication even in the absence of NO production suggests a novel bacteriostatic mechanism. As H+-ATPases do participate in vesicular trafficking and interact with GTPases (26, 33, 41), we cannot formally exclude a more direct interference of omeprazole with vesicular trafficking and, hence, interference with the SPI2-directed formation of the salmonella-containing vacuoles and bacterial intracellular replication.
S. Typhimurium has itself been reported to suppress NO production in infected murine macrophage J774.A.1 cells (4, 10). This effect on J774A.1 cells, however, did not involve suppression in NF-κB signaling or iNOS expression (10). This further implies that the effect of omeprazole on NO production did not directly relate to S. Typhimurium infection itself but rather to an interference with the expression or release of inflammatory mediators. Indeed, we could demonstrate that omeprazole likewise inhibited iNOS expression and the release of NO, TNF-α, and IL-6 in responses to purified S. Typhimurium Ra lipopolysaccharide (data not shown).
Bafilomycin A1, which has been reported to mediate the killing of S. Typhimurium in RAW264.7 cells (35), increased the NO production in infected cells. In principle, therefore, the enhanced antibacterial potential of bafilomycin A1 could have originated from its ability to prime RAW264.7 cells for the production of NO. However, the potential cytotoxicity observed here for bafilomycin A1 also could affect the viability of intracellular bacteria. For example, the disruption of the membrane integrity of the host cell might lead to the exposure of intracellular bacteria to gentamicin, which is used for preventing extracellular bacterial replication.
Thus, proton pump inhibitors can be added to the list of compounds that interfere with bacterial virulence under experimental settings; however, separate proton pump inhibitors differentially interfere with bacterial virulence-associated fitness.
Acknowledgments
This work was supported by grants from the Swedish Research Council.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Beuzón, C. R., G. Banks, J. Deiwick, M. Hensel, and D. W. Holden. 1999. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol. Microbiol. 33:806-816. [DOI] [PubMed] [Google Scholar]
- 2.Beuzón, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjur, E., S. Eriksson-Ygberg, F. Aslund, and M. Rhen. 2006. Thioredoxin 1 promotes intracellular replication and virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 74:5140-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjur, E., S. Eriksson-Ygberg, and M. Rhen. 2006. The O-antigen affects replication of Salmonella enterica serovar Typhimurium in murine macrophage-like J774-A.1 cells through modulation of host cell nitric oxide production. Microbes Infect. 8:1826-1838. [DOI] [PubMed] [Google Scholar]
- 5.Capodicasa, E., P. Cornacchione, B. Natalini, A. Bartoli, S. Coaccioli, P. Marconi, and L. Scaringi. 2008. Omeprazole induces apoptosis in normal human polymorphonuclear leucocytes. Int. J. Immunopathol. Pharmacol. 21:73-85. [DOI] [PubMed] [Google Scholar]
- 6.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravortty, D., M. Rohde, L. Jager, J. Deiwick, and M. Hensel. 2005. Formation of a novel surface structure encoded by Salmonella pathogenicity island 2. EMBO J. 24:2043-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Milito, A., E. Iessi, M. Logozzi, F. Lozupone, M. Spada, M. L. Marino, C. Federici, M. Perdicchio, P. Matarrese, L. Lugini, A. Nilsson, and S. Fais. 2007. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 67:5408-5417. [DOI] [PubMed] [Google Scholar]
- 9.Dramsi, S., and P. Cossart. 2002. Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite. J. Cell Biol. 156:943-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson, S., J. Bjorkman, S. Borg, A. Syk, S. Pettersson, D. I. Andersson, and M. Rhen. 2000. Salmonella typhimurium mutants that downregulate phagocyte nitric oxide production. Cell Microbiol. 2:239-250. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 12.Freeman, J. A., M. E. Ohl, and S. I. Miller. 2003. The Salmonella enterica serovar Typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect. Immun. 71:418-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galán, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier, A., M. L. Robertson, M. Lowden, J. A. Ibarra, J. L. Puente, and B. B. Finlay. 2005. Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli. Antimicrob. Agents Chemother. 49:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen-Wester, I., and M. Hensel. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3:549-559. [DOI] [PubMed] [Google Scholar]
- 16.Hautefort, I., M. J. Proenca, and J. C. Hinton. 2003. Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl. Environ. Microbiol. 69:7480-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinoki, A., K. Yoshimura, K. Fujita, M. Akita, R. Ikeda, M. Nagashima, M. Nomura, and A. Satomi. 2006. Suppression of proinflammatory cytokine production in macrophages by lansoprazole. Pediatr. Surg. Int. 22:915-923. [DOI] [PubMed] [Google Scholar]
- 18.Hong, J., Y. Nakano, A. Yokomakura, K. Ishihara, S. Kim, Y. S. Kang, and K. Ohuchi. 2006. Nitric oxide production by the vacuolar-type (H+)-ATPase inhibitors bafilomycin A1 and concanamycin A and its possible role in apoptosis in RAW 264.7 cells. J. Pharmacol. Exp. Ther. 319:672-681. [DOI] [PubMed] [Google Scholar]
- 19.Hung, D. T., and E. J. Rubin. 2006. Chemical biology and bacteria: not simply a matter of life or death. Curr. Opin. Chem. Biol. 10:321-326. [DOI] [PubMed] [Google Scholar]
- 20.Hung, D. T., E. A. Shakhnovich, E. Pierson, and J. J. Mekalanos. 2005. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310:670-674. [DOI] [PubMed] [Google Scholar]
- 21.Kauppi, A. M., R. Nordfelth, H. Uvell, H. Wolf-Watz, and M. Elofsson. 2003. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem. Biol. 10:241-249. [DOI] [PubMed] [Google Scholar]
- 22.Keyser, P., M. Elofsson, S. Rosell, and H. Wolf-Watz. 2008. Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against gram-negative bacteria. J. Intern. Med. 264:17-29. [DOI] [PubMed] [Google Scholar]
- 23.Kuhle, V., and M. Hensel. 2004. Cellular microbiology of intracellular Salmonella enterica: functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell Mol. Life. Sci. 61:2812-2826. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Löber, S., D. Jackel, N. Kaiser, and M. Hensel. 2006. Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals. Int. J. Med. Microbiol. 296:435-447. [DOI] [PubMed] [Google Scholar]
- 26.Marshansky, V., and M. Futai. 2008. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr. Opin. Cell Biol. 20:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merlen, C., D. Fayol-Messaoudi, S. Fabrega, T. El Hage, A. Servin, and F. Authier. 2005. Proteolytic activation of internalized cholera toxin within hepatic endosomes by cathepsin D. FEBS J. 272:4385-4397. [DOI] [PubMed] [Google Scholar]
- 28.Miguel, D. C., J. K. Yokoyama-Yasunaka, W. K. Andreoli, R. A. Mortara, and S. R. Uliana. 2007. Tamoxifen is effective against Leishmania and induces a rapid alkalinization of parasitophorous vacuoles harbouring Leishmania (Leishmania) amazonensis amastigotes. J. Antimicrob. Chemother. 60:526-534. [DOI] [PubMed] [Google Scholar]
- 29.Morimura, T., K. Fujita, M. Akita, M. Nagashima, and A. Satomi. 2008. The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr. Surg. Int. 24:1087-1094. [DOI] [PubMed] [Google Scholar]
- 30.Muschiol, S., L. Bailey, A. Gylfe, C. Sundin, K. Hultenby, S. Bergstrom, M. Elofsson, H. Wolf-Watz, S. Normark, and B. Henriques-Normark. 2006. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 103:14566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negrea, A., E. Bjur, S. E. Ygberg, M. Elofsson, H. Wolf-Watz, and M. Rhen. 2007. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 51:2867-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordfelth, R., A. M. Kauppi, H. A. Norberg, H. Wolf-Watz, and M. Elofsson. 2005. Small-molecule inhibitors specifically targeting type III secretion. Infect. Immun. 73:3104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palokangas, H., M. Ying, K. Vaananen, and J. Saraste. 1998. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell 9:3561-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pósfai, G., M. D. Koob, H. A. Kirkpatrick, and F. R. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathman, M., M. D. Sjaastad, and S. Falkow. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhen, M., and C. J. Dorman. 2005. Hierarchical gene regulators adapt Salmonella enterica to its host milieus. Int. J. Med. Microbiol. 294:487-502. [DOI] [PubMed] [Google Scholar]
- 37.Steele-Mortimer, O. 2008. Infection of epithelial cells with Salmonella enterica. Methods Mol. Biol. 431:201-211. [DOI] [PubMed] [Google Scholar]
- 38.Steele-Mortimer, O., M. St.-Louis, M. Olivier, and B. B. Finlay. 2000. Vacuole acidification is not required for survival of Salmonella enterica serovar Typhimurium within cultured macrophages and epithelial cells. Infect. Immun. 68:5401-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun-Wada, G. H., Y. Wada, and M. Futai. 2004. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim. Biophys. Acta 1658:106-114. [DOI] [PubMed] [Google Scholar]
- 40.Tapper, H., and R. Sundler. 1995. Bafilomycin A1 inhibits lysosomal, phagosomal, and plasma membrane H+-ATPase and induces lysosomal enzyme secretion in macrophages. J. Cell Physiol. 163:137-144. [DOI] [PubMed] [Google Scholar]
- 41.van Weert, A. W., H. J. Geuze, and W. Stoorvogel. 1997. Heterogeneous behavior of cells with respect to induction of retrograde transport from the trans-Golgi network to the Golgi upon inhibition of the vacuolar proton pump. Eur. J. Cell Biol. 74:417-423. [PubMed] [Google Scholar]
- 42.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson, L. C., and E. A. Neale. 1994. Bafilomycin A1 inhibits the action of tetanus toxin in spinal cord neurons in cell culture. J. Neurochem. 63:2342-2345. [DOI] [PubMed] [Google Scholar]
- 44.Wolf, K., H. J. Betts, B. Chellas-Gery, S. Hower, C. N. Linton, and K. A. Fields. 2006. Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle. Mol. Microbiol. 61:1543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, J., H. T. Feng, C. Wang, K. H. Yip, N. Pavlos, J. M. Papadimitriou, D. Wood, and M. H. Zheng. 2003. Effects of bafilomycin A1: an inhibitor of vacuolar H+-ATPases on endocytosis and apoptosis in RAW cells and RAW cell-derived osteoclasts. J. Cell Biochem. 88:1256-1264. [DOI] [PubMed] [Google Scholar]