Abstract
The recombinant industrial Saccharomyces cerevisiae strain MA-R5 was engineered to express NADP+-dependent xylitol dehydrogenase using the flocculent yeast strain IR-2, which has high xylulose-fermenting ability, and both xylose consumption and ethanol production remarkably increased. Furthermore, the MA-R5 strain produced the highest ethanol yield (0.48 g/g) from nonsulfuric acid hydrolysate of wood chips.
Successful fermentation of lignocellulosic biomass to ethanol is dependent on efficient utilization of d-xylose, which is the second most common fermentable sugar in the hydrolysate. Although the well-known fermentative yeast Saccharomyces cerevisiae is one of the most effective ethanol-producing organisms for hexose sugars, it is not able to ferment d-xylose. However, S. cerevisiae can metabolize an isomerization product of d-xylose, d-xylulose, which is phosphorylated to d-xylulose 5-phosphate, channeled through the pentose phosphate pathway and glycolysis.
S. cerevisiae transformed with the XYL1 and XYL2 genes encoding xylose reductase (XR) and xylitol dehydrogenase (XDH) from Pichia stipitis (referred to as PsXR and PsXDH, respectively) acquires the ability to ferment d-xylose to ethanol (2, 5, 6, 9, 10, 12, 22). Furthermore, overexpression of the XKS1 gene encoding xylulokinase (XK) from S. cerevisiae (ScXK) has been shown to aid d-xylose utilization (4, 7, 11, 16, 23), with xylitol still a major by-product. Kuyper et al. (14) also demonstrated the successful fermentation of d-xylose to ethanol using recombinant S. cerevisiae strains expressing fungal xylose isomerase. However, these approaches are insufficient for industrial bioprocesses, mainly due to the low rate of d-xylose fermentation.
Xylitol excretion has been ascribed mainly to the difference in coenzyme specificities between PsXR (with NADPH) and PsXDH (with NAD+), which creates an intracellular redox imbalance (1). Therefore, modifying the coenzyme specificity of XR and/or XDH by protein engineering is an attractive approach for achieving efficient fermentation of ethanol from d-xylose using recombinant S. cerevisiae. Watanabe et al. (24) previously succeeded in generating several PsXDH mutants (e.g., quadruple ARSdR mutant) with a complete reversal of coenzyme specificity toward NADP+ by multiple site-directed mutagenesis on amino acids from the coenzyme-binding domain. The ARSdR mutant (D207A/I208R/F209S/N211R) has more that 4,500-fold-higher catalytic efficiency (kcat/Km) with NADP+ than the wild-type PsXDH. In addition, we recently found that several laboratory recombinant S. cerevisiae strains, in which the ARSdR mutant, along with PsXR and ScXK, is expressed through a strong constitutive promoter, increased ethanol production from d-xylose at the expense of xylitol excretion (17, 18), probably by maintaining the intracellular redox balance. However, commercialization of lignocellulosic hydrolysate fermentation requires industrial strains that are more robust than laboratory strains (5, 19, 21).
A potential host for developing d-xylose-fermenting strains requires an active and efficient pentose phosphate pathway linking the d-xylose-to-d-xylulose pathway to glycolysis. In the case of S. cerevisiae, strains have different d-xylulose fermentation abilities (3, 25), indicating inherent differences in the capacities of these strains to ferment pentose sugars. Furthermore, anaerobic d-xylulose fermentation was investigated to identify genetic backgrounds potentially beneficial to anaerobic d-xylose fermentation in strains not exhibiting product formation related to the redox imbalance generated by the first two steps of the eukaryotic d-xylose metabolism (3), although the physiological purpose of the different d-xylulose fermentation abilities of S. cerevisiae is not yet clear. Therefore, we selected an efficient industrial d-xylulose-fermenting S. cerevisiae strain as a host for constructing a recombinant strain through chromosomal integration of the NADP+-dependent XDH gene and the XR and endogenous XK genes. Using this recombinant strain, we characterized the enzyme activity and ability to ferment both d-xylose and lignocellulosic hydrolysate.
d-Xylulose fermentation using industrial S. cerevisiae strains.
We compared anaerobic d-xylulose fermentation among nine different commercially available industrial diploid strains of S. cerevisiae to identify genetic modifications suitable for anaerobic d-xylose fermentation (Fig. 1). These industrial strains include bakery yeast (Type II), shochu yeasts (S3 and S2), wine yeasts (W1 and W4), and industrial alcohol fermentation yeasts (IFO 0224, FERM I-6, FERM I-7, and IR-2) (Table 1). For d-xylulose fermentation experiments, the yeast strains were first cultivated aerobically in YPD medium (10 g liter−1 yeast extract, 20 g liter−1 peptone, and 20 g liter−1 glucose) for 24 h at 30°C. Then, 0.5-ml aliquots of the precultures were transferred to 4.5 ml YPGX medium (50 g liter−1 glucose and 27 g l−1 d-xylulose supplemented with 10 g liter−1 yeast extract and 20 g liter−1 peptone) and fermented at 30°C in closed bottles (13 ml) with mild agitation (100 rpm). d-Xylulose was prepared by the method of Olsson et al. (20). Samples (0.1 ml) of the fermentation broth were removed at intervals using a syringe and diluted 10 times with sterile water. Monosaccharides and alcohols were determined by high-performance liquid chromatography (8).
FIG. 1.
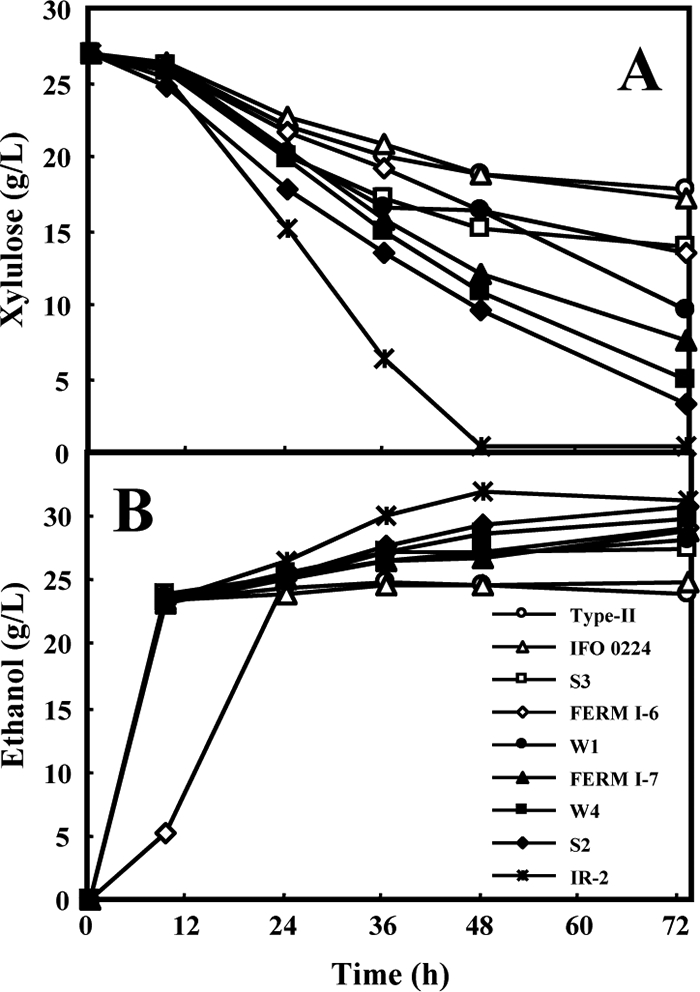
Time-dependent batch fermentation profiles of d-xylulose consumption (A) or ethanol production (B) by industrial yeast strains grown in YPGX medium containing glucose (50 g liter−1) and d-xylulose (27 g liter−1) under anaerobic conditions. Small amounts of glycerol (<3.2 g liter−1) and acetic acid (<1.8 g liter−1) were detected, mainly during the glucose consumption phase (data not shown).
TABLE 1.
Characteristics of S. cerevisiae strains and plasmids used in this study
| Strain/plasmid | Relevant genotype | Source and/or reference |
|---|---|---|
| S. cerevisiae strains | ||
| Type II | Bakery yeast, MATa/α | Sigma-Aldrich |
| IFO 0224 | Alcohol-fermenting yeast, MATa/α | National Institute of Technology and Evaluation |
| S3 | Shochu yeast no. 3, MATa/α | Brewing Society of Japan |
| FERM I-6 | Alcohol-fermenting yeast, MATa/α | Advanced Industrial Science and Technology |
| W1 | Wine yeast no. 1, MATa/α | Brewing Society of Japan |
| FERM I-7 | Alcohol-fermenting yeast, MATa/α | Advanced Industrial Science and Technology |
| W4 | Wine yeast no. 4, MATa/α | Brewing Society of Japan |
| S2 | Shochu yeast no. 2, MATa/α | Brewing Society of Japan |
| IR-2 | Alcohol-fermenting flocculent yeast, MATa/α | Advanced Industrial Science and Technology; 13 |
| MA-R1 | IR-2, AUR1 | 15 |
| MA-R4 | IR-2, AUR1::AUR1-C-[PGKp-XK-PGKt, PGKp-XDH-PGKt, PGKp-XR-PGKt] | 15 |
| MA-R5 | IR-2, AUR1::AUR1-C-[PGKp-XK-PGKt, PGKp-XDH(ARSdR)-PGKt, PGKp-XR-PGKt] | This study |
| Plasmids | ||
| pAUR101 | AUR1 (control plasmid) | Takara Bio |
| pAUR-XKXDHXR | AUR-C expression of XK, wild-type XDH, and XR genes | 17 |
| pAUR-XKARSdRXR | AUR-C expression of XK, mutated XDH, and XR genes | 17 |
Almost all yeast strains initially consumed glucose within 9 h (data not shown). The well-characterized, flocculent S. cerevisiae strain IR-2, isolated from Indonesian fermented food (13), metabolized d-xylulose very quickly (Fig. 1A), consuming 98% of the d-xylulose at 48 h, with the highest xylulose consumption rate of 0.55 g liter−1 h−1. Furthermore, the IR-2 strain produced the largest amount of ethanol at 32.0 g liter−1 (Fig. 1B), with a yield of 0.43 g of ethanol per gram of total consumed sugars (glucose and d-xylulose) (g/g). Therefore, IR-2 was selected as a host strain for genetically engineering d-xylose fermentation.
Construction of recombinant yeasts.
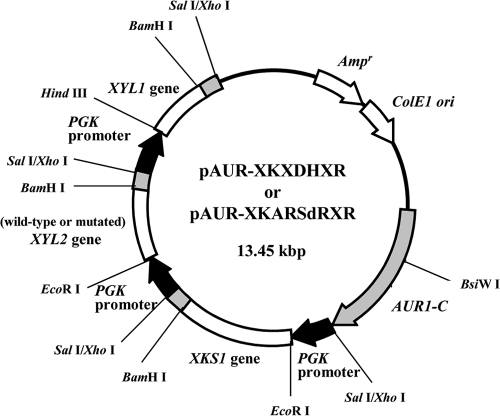
We constructed two chromosome-integrating expression plasmids using pAUR101 (Takara Bio, Kyoto, Japan) with PGK promoters to express PsXR (XYL1), wild-type or NADP+-dependent PsXDH (XYL2), and ScXK (XKS1) (Fig. 2); the method for constructing the plasmids, which can be transformed into any S. cerevisiae strain, was previously described in detail (17). The plasmids pAUR-XKXDHXR, pAUR-XKARSdRXR, and pAUR101, all digested with BsiWI, were transformed into IR-2 to construct the recombinant strains MA-R4 and MA-R5 and the control strain MA-R1, respectively (Table 1); each transformant was selected from YPD medium plates containing 0.5 mg liter−1 aureobasidin A (Takara Bio).
FIG. 2.
Map of pAUR-XKXDHXR for coexpression of PsXR, wild-type PsXDH, and ScXK or pAUR-XKARSdRXR for coexpression of PsXR, mutated PsXDH (ARSdR), and ScXK.
Enzyme activity.
MA-R1, MA-R4, and MA-R5 were examined for XR, XDH, and XK activities (Table 2). The activities of these d-xylose metabolic enzymes were determined with freshly prepared cell extracts as described previously (17). Briefly, MA-R1 was cultured in YPD medium, and MA-R4 and MA-R5 were cultured in YPDX medium (10 g liter−1 yeast extract, 20 g liter−1 peptone, 10 g liter−1 glucose, and 10 g liter−1 d-xylose) for 24 h at 30°C. The harvested cells were suspended in the yeast protein extraction reagent Y-PER (Pierce, Rockford, IL) and disrupted by agitation using a mixer to obtain the supernatants as cell extracts. Protein concentrations in the cell extracts were determined using a Micro-BCA protein assay kit (Pierce). MA-R5 showed more than 240-fold-higher XDH activity with NADP+ than did MA-R1, while MA-R4 showed more than 200-fold-higher XDH activity with NAD+ than did MA-R1. The activities of XR and XK were similarly high in the MA-R4 and MA-R5 strains. Furthermore, these strains exhibited stable recombinant enzyme activities and could be cultured in nonselective medium (YPD medium) without a significant loss of d-xylose-fermenting ability for more than 20 generations (data not shown).
TABLE 2.
Activities of XR, XDH, and XK in cell extracts of recombinant yeast strainsa
| Strain | Activity (U/mg) of enzyme
|
|||
|---|---|---|---|---|
| XR | XDH
|
XK | ||
| NAD+ | NADP+ | |||
| MA-R1 | 0.018 ± 0.003 | 0.006 ± 0.001 | 0.003 ± 0.001 | 0.031 ± 0.004 |
| MA-R4 | 0.703 ± 0.132 | 1.229 ± 0.271 | 0.002 ± 0.001 | 0.116 ± 0.019 |
| MA-R5 | 0.796 ± 0.267 | 0.039 ± 0.017 | 0.735 ± 0.103 | 0.118 ± 0.004 |
Values are the averages for three independent experiments ± the standard deviations.
Effect of modifying coenzyme specificity on d-xylose fermentation.
Ethanol production of MA-R4 and MA-R5 was examined in YPX medium (10 g liter−1 yeast extract, 20 g liter−1 peptone, and 45 g liter−1 d-xylose) for 72 h at 30°C as described previously (15). MA-R4 and MA-R5 consumed 79% and 95% of the d-xylose at 33 h, respectively (Fig. 3); MA-R5 produced a maximum of 16.0 g liter−1 ethanol at 48 h, while MA-R4 produced no more than 15.3 g liter−1 (Fig. 3). MA-R5 exhibited a 39%-higher xylose consumption rate and a 39%-higher ethanol production rate than MA-R4 (Table 3); these rates for MA-R5 were significantly different compared to those for MA-R4 (P = 0.011 and 0.032, respectively). Moreover, the ethanol yield of MA-R5 was higher than that of MA-R4 but not significantly (P = 0.052). The ethanol yields of MA-R5 and MA-R4 correspond to 72.6% and 65.7% of the theoretical yield (0.51 g of ethanol per gram of consumed xylose), respectively. In contrast, the xylitol, glycerol, and acetate yields were lower for MA-R5 (0.038 ± 0.000, 0.076 ± 0.009, and 0.007 ± 0.001 g/g, respectively) than in MA-R4 (0.048 ± 0.004, 0.101 ± 0.001, and 0.014 ± 0.001 g/g, respectively); these yields for MA-R5 were significantly different from those for MA-R4 (P = 0.042, 0.048, and 0.020, respectively). Thus, the higher ethanol yield of MA-R5 may be directly related to the low by-product yields.
FIG. 3.
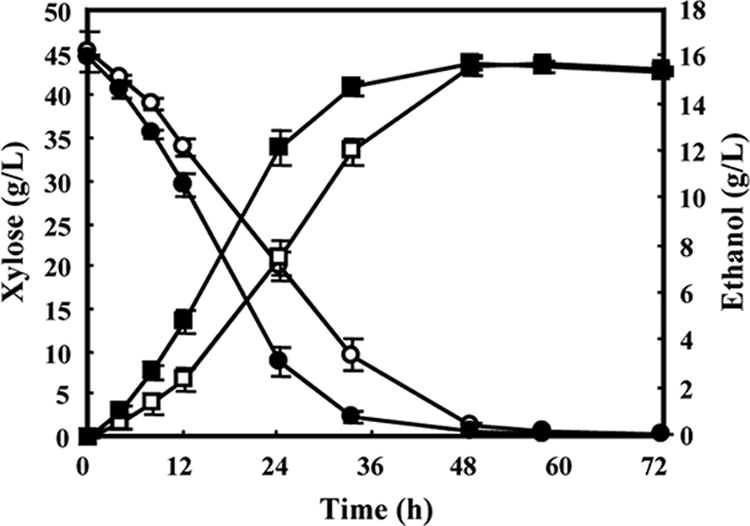
Time-dependent batch fermentation profiles of d-xylose consumption (circles) and ethanol production (squares) by recombinant S. cerevisiae, MA-R4 (open symbols) or MA-R5 (closed symbols), in YPX medium containing d-xylose (45 g liter−1) under anaerobic conditions. Small amounts of xylitol, glycerol, and acetic acid are not shown (see the text). Data points represent the averages for two independent experiments.
TABLE 3.
Xylose consumption rates, ethanol production rates, and ethanol yields of recombinant S. cerevisiae strains in fermentation of d-xylosea
| Strain | Xylose consumption rate (g liter−1 h−1) | Ethanol production rate (g liter−1 h−1) | Ethanol yieldb |
|---|---|---|---|
| MA-R4 | 1.07 ± 0.02 | 0.36 ± 0.02 | 0.34 ± 0.00 |
| MA-R5 | 1.49 ± 0.06 | 0.50 ± 0.03 | 0.37 ± 0.01 |
Values are the averages ± the standard deviations of two independent experiments.
Ethanol yield is expressed in grams of produced ethanol per gram of consumed xylose after 48 h of fermentation.
Fermentation analysis of lignocellulosic hydrolysate.
Finally, to test whether MA-R5 can indeed efficiently ferment mixed sugars containing glucose and d-xylose of hydrolysate from lignocellulosic biomass to ethanol, lignocellulosic eucalyptus-based hydrolysate was fermented using MA-R5 together with the control strain MA-R1 in medium containing 62.5 g liter−1 glucose, 1.2 g liter−1 mannose, 2.1 g liter−1 galactose, 13.2 g liter−1 d-xylose, and 0.6 g liter−1 l-arabinose (Fig. 4). Lignocellulosic hydrolysate was prepared through the ball milling (BM) treatment of eucalyptus wood chips, followed by enzymatic hydrolysis, as reported previously (8). Briefly, the eucalyptus samples were pretreated for a total of 240 min (a cycle of 10 min of milling and 10 min of pausing) by BM using a Pulverisette 7 planetary mill (Fritsch, Germany), and then 20-g pretreated samples were incubated in 50 mM acetate buffer (pH 5.0) containing 100 ml cellulase mixture (40 FPU/g substrate) at 45°C for 72 h. The resulting hydrolysate was adjusted to pH 5.5 using NaOH, and then 10 g liter−1 yeast extract was added for fermentation performance analysis. BM-treated eucalypti are hydrolyzed at lower enzymatic levels and have no inhibitory effect on enzymatic hydrolysis or ethanol fermentation (8).
FIG. 4.
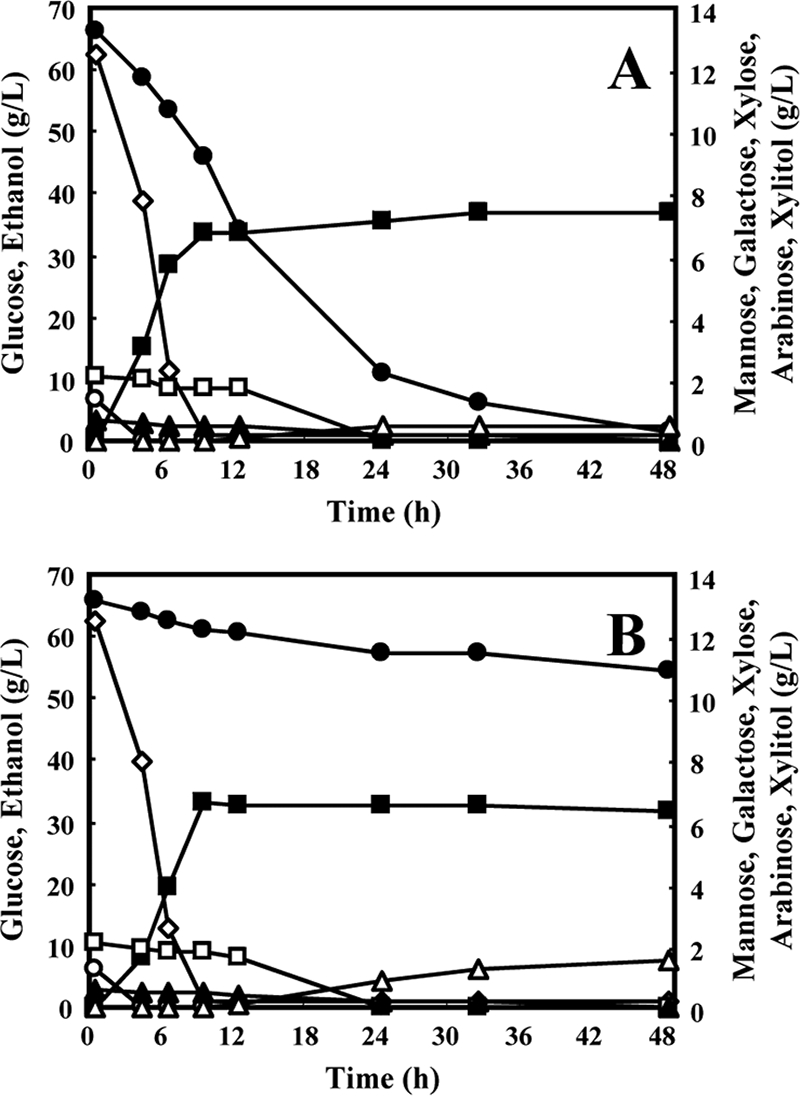
Time-dependent batch fermentation profiles of MA-R5 (A) or MA-R1 (B) in lignocellulosic hydrolysate under anaerobic conditions. Glucose, open rhomboids; mannose, open circles; galactose, open squares; d-xylose, filled circles; l-arabinose, filled triangles; xylitol, open triangles; ethanol, filled squares. The small amount of glycerol is not shown (see the text). Data points represent the averages of two independent experiments. Error bars are not shown, but deviations were below 10% of the average.
Both MA-R5 and the control strain (MA-R1) rapidly fermented glucose within 9 h and mannose within 4 h while slowly consuming galactose within 24 h (Fig. 4A and B); interestingly, both strains consumed l-arabinose within 48 h (Fig. 4A and B). MA-R5 fermented 84% of the d-xylose in 24 h and metabolized almost all of the d-xylose after 48 h of fermentation (Fig. 4A), whereas the control strain hardly metabolized d-xylose (Fig. 4B). Furthermore, MA-R5 was able to efficiently coferment glucose and d-xylose of hydrolysate to ethanol simultaneously (0 to 9 h; Fig. 4A). The highest ethanol production by MA-R5 reached approximately 37.6 g liter−1 after 48 h of fermentation, which was higher than that of the control strain (<33.2 g liter−1). MA-R5 showed a xylose consumption rate of 0.44 ± 0.03 g liter−1 h−1 and an ethanol production rate of 1.49 ± 0.01 g liter−1 h−1. After 48 h of fermentation, the ethanol yields per gram of total consumed sugars (g/g) of MA-R5 and the control strain were 0.48 ± 0.01 g/g and 0.46 ± 0.00 g/g, corresponding to 93.2% and 90.0% of the theoretical yield, respectively. MA-R5 excreted less xylitol (0.51 g liter−1) at 48 h than the control strain (1.54 g liter−1) (Fig. 4A and B); the xylitol yield from consumed d-xylose by MA-R5 was low (0.039 ± 0.001 g/g). The same low glycerol yield of 0.023 ± 0.002 g/g from total consumed sugars was obtained with both strains.
Conclusions.
Having the best performance in d-xylulose-to-ethanol conversion among the industrial S. cerevisiae strains, the flocculent strain IR-2 was used as the host for genetically engineering d-xylose fermentation. The recombinant strain MA-R5, expressing the protein-engineered NADP+-dependent PsXDH gene, markedly increased the xylose consumption rate and showed a high ethanol yield compared with the reference strain, MA-R4. Furthermore, MA-R5 was able to effectively coferment glucose and d-xylose and to produce ethanol at a high yield (0.48 g/g of consumed sugars) from lignocellulosic hydrolysate. These results demonstrate that the use of this redox-engineered strain has several advantages for scaling up production of ethanol from lignocellulosic hydrolysates.
Acknowledgments
We thank Katsuji Murakami and Osamu Takimura (AIST) for their kind gift of lignocellulosic hydrolysates and their valuable discussions and Maiko Kato for her technical assistance.
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Footnotes
Published ahead of print on 27 March 2009.
REFERENCES
- 1.Bruinenberg, P. M., P. H. M. de Bot, J. P. van Dijken, and W. A. Scheffers. 1983. The role of redox balances in the anaerobic fermentation of xylose by yeasts. Appl. Microbiol. Biotechnol. 18:287-292. [Google Scholar]
- 2.Chu, B. C., and H. Lee. 2007. Genetic improvement of Saccharomyces cerevisiae for xylose fermentation. Biotechnol. Adv. 25:425-441. [DOI] [PubMed] [Google Scholar]
- 3.Eliasson, A., E. Boles, B. Johansson, M. Österberg, J. M. Thevelein, I. Spencer-Martins, H. Juhnke, and B. Hahn-Hägerdal. 2000. Xylulose fermentation by mutant and wild-type strains of Zygosaccharomyces and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 53:376-382. [DOI] [PubMed] [Google Scholar]
- 4.Eliasson, A., C. Christensson, C. F. Wahlbom, and B. Hahn-Hägerdal. 2000. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl. Environ. Microbiol. 66:3381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn-Hägerdal, B., K. Karhumaa, C. Fonseca, I. Spencer-Martins, and M. F. Gorwa-Grauslund. 2007. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 74:937-953. [DOI] [PubMed] [Google Scholar]
- 6.Hahn-Hägerdal, B., K. Karhumaa, M. Jeppsson, and M. F. Gorwa-Grauslund. 2007. Metabolic engineering for pentose utilization in Saccharomyces cerevisiae. Adv. Biochem. Eng. Biotechnol. 108:147-177. [DOI] [PubMed] [Google Scholar]
- 7.Ho, N. W. Y., Z. Chen, and A. P. Brainard. 1998. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 64:1852-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue, H., S. Yano, T. Endo, T. Sakaki, and S. Sawayama. 2008. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffries, T. W. 2006. Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol. 17:320-326. [DOI] [PubMed] [Google Scholar]
- 10.Jeffries, T. W., and Y. S. Jin. 2004. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol. 63:495-509. [DOI] [PubMed] [Google Scholar]
- 11.Johansson, B., C. Christensson, T. Hobley, and B. Hahn-Hägerdal. 2001. Xylulokinase overexpression in two strains of Saccharomyces cerevisiae also expressing xylose reductase and xylitol dehydrogenase and its effect on fermentation of xylose and lignocellulosic hydrolysate. Appl. Environ. Microbiol. 67:4249-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kötter, P., and M. Ciriacy. 1993. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 38:776-783. [Google Scholar]
- 13.Kuriyama, H., Y. Seiko, T. Murakami, H. Kobayashi, and Y. Sonoda. 1985. Continuous ethanol fermentation with cell recycling using flocculating yeast. J. Ferment. Technol. 63:159-165. [Google Scholar]
- 14.Kuyper, M., H. R. Harhangi, A. K. Stave, A. A. Winkler, M. S. Jetten, W. T. De Laat, J. J. Den Ridder, H. J. Op den Camp, J. P. Van Dijken, and J. T. Pronk. 2003. High-level functional expression of a fungal xylose isomerase: the key to efficient ethanolic fermentation of xylose by Saccharomyces cerevisiae? FEMS Yeast Res. 4:69-78. [DOI] [PubMed] [Google Scholar]
- 15.Matsushika, A., H. Inoue, K. Murakami, O. Takimura, and S. Sawayama. 2009. Bioethanol production performance of five recombinant strains of laboratory and industrial xylose-fermenting Saccharomyces cerevisiae. Bioresour. Technol. 100:2392-2398. [DOI] [PubMed] [Google Scholar]
- 16.Matsushika, A., and S. Sawayama. 2008. Efficient bioethanol production from xylose by recombinant Saccharomyces cerevisiae requires high activity of xylose reductase and moderate xylulokinase activity. J. Biosci. Bioeng. 106:306-309. [DOI] [PubMed] [Google Scholar]
- 17.Matsushika, A., S. Watanabe, T. Kodaki, K. Makino, H. Inoue, K. Murakami, O. Takimura, and S. Sawayama. 2008. Expression of protein engineered NADP+-dependent xylitol dehydrogenase increases ethanol production from xylose in recombinant Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 81:243-255. [DOI] [PubMed] [Google Scholar]
- 18.Matsushika, A., S. Watanabe, T. Kodaki, K. Makino, and S. Sawayama. 2008. Bioethanol production from xylose by recombinant Saccharomyces cerevisiae expressing xylose reductase, NADP+-dependent xylitol dehydrogenase, and xylulokinase. J. Biosci. Bioeng. 105:296-299. [DOI] [PubMed] [Google Scholar]
- 19.Olsson, L., and B. Hahn-Hägerdal. 1993. Fermentative performance of bacteria and yeasts in lignocellulose hydrolysates. Process Biochem. 28:249-257. [Google Scholar]
- 20.Olsson, L., T. Lindén, and B. Hahn-Hägerdal. 1994. A rapid chromatographic method for the production of preparative amounts of xylulose. Enzyme Microb. Technol. 16:388-394. [Google Scholar]
- 21.Sonderegger, M., M. Jeppsson, C. Larsson, M. F. Gorwa-Grauslund, E. Boles, L. Olsson, I. Spencer-Martins, B. Hahn-Hägerdal, and U. Sauer. 2004. Fermentation performance of engineered and evolved xylose-fermenting Saccharomyces cerevisiae strains. Biotechnol. Bioeng. 87:90-98. [DOI] [PubMed] [Google Scholar]
- 22.Tantirungkij, M., N. Nakashima, T. Seki, and T. Yoshida. 1993. Construction of xylose-assimilating Saccharomyces cerevisiae. J. Ferment. Bioeng. 75:83-88. [Google Scholar]
- 23.Toivari, M. H., A. Aristidou, L. Ruohonen, and M. Penttilä. 2001. Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability. Metab. Eng. 3:236-249. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, S., T. Kodaki, and K. Makino. 2005. Complete reversal of coenzyme specificity of xylitol dehydrogenase and increase of thermostability by the introduction of structural zinc. J. Biol. Chem. 280:10340-10349. [DOI] [PubMed] [Google Scholar]
- 25.Yu, S., H. Jeppsson, and B. Hahn-Hägerdal. 1995. Xylulose fermentation by Saccharomyces cerevisiae and xylose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 44:314-320. [DOI] [PubMed] [Google Scholar]