FIG. 2.
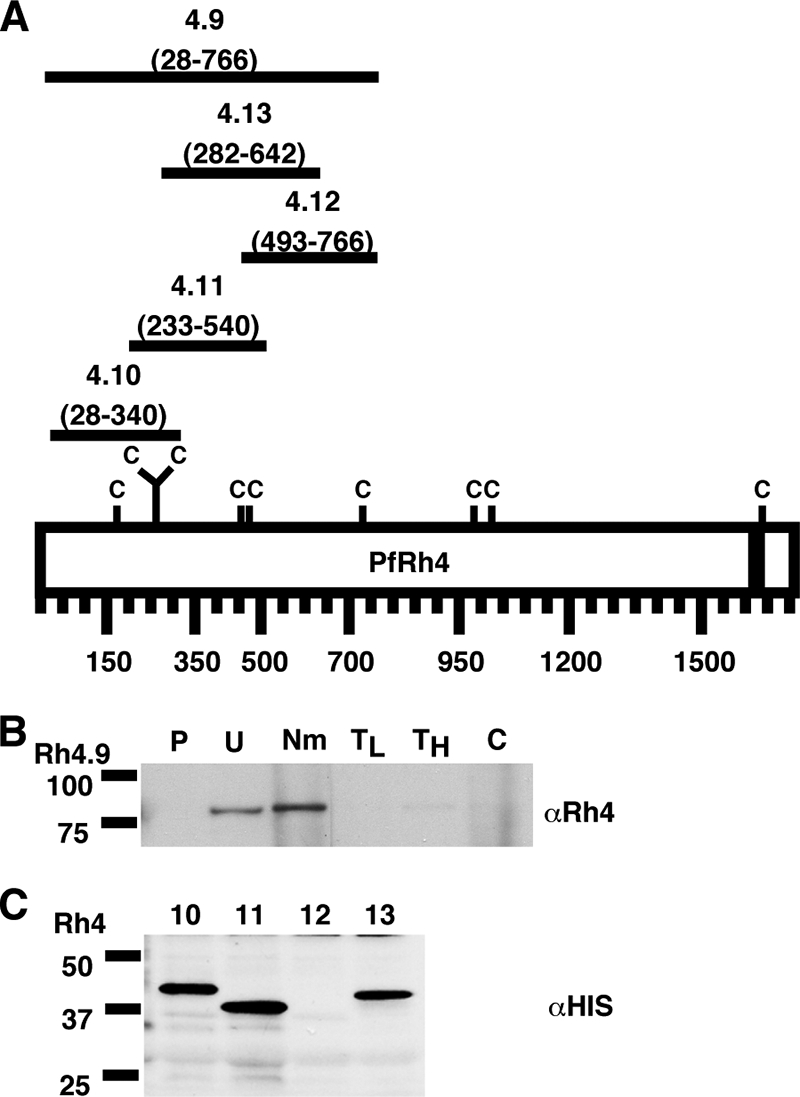
PfRh4 binds to the erythrocyte surface through its N-terminal region. (A) Schematic representation of the various six-His-tagged PfRh4 recombinant proteins. The C denotes cysteine residues, and the black bar represents the transmembrane domain of PfRh4. The number below each fusion protein indicates the amino acid sequence that it encompasses. (B) rRh4.9 binds erythrocytes in a manner similar to that of native PfRh4. Immunodetection of the recombinant fusion protein with anti-Rh4 (αRh4) antibodies after binding and elution from untreated and enzyme-treated erythrocytes is shown. Lanes: P, proteins eluted from PBS control; U, untreated erythrocytes; Nm, neuraminidase; TL, low trypsin; TH, high trypsin; and C, chymotrypsin-treated erythrocytes. Low trypsin and high tryspin refer to trypsin treatments with 0.1 and 1.5 mg/ml of enzyme, respectively. Molecular masses are indicated on the left (in kDa). (C) Minimal binding domain of PfRh4. Binding of six-His-tagged recombinant PfRh4 proteins (Rh4.10, Rh4.11, Rh4.12, and Rh4.13) to untreated erythrocytes was detected using mouse monoclonal anti-His5 (αHis) antibodies. Molecular masses are indicated on the left (in kDa).
