FIG. 5.
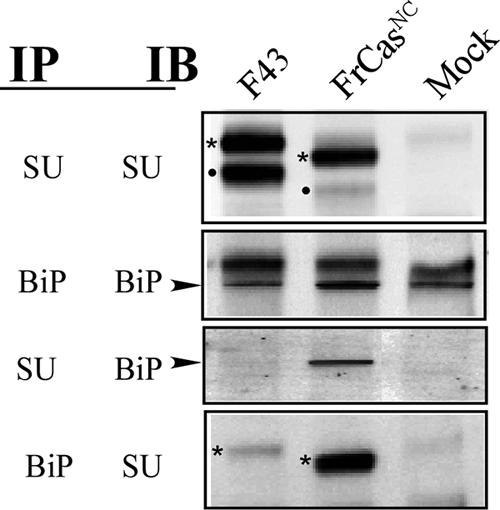
Folding stability of the envelope proteins of FrCasNC and F43 were assessed by the extent of binding of the ER chaperone BiP. NIH 3T3 cells were infected with F43 or FrCasNC, and the cells were lysed 48 h later. Lysates were immunoprecipitated (IP) with anti-SU or anti-BiP (anti-KDEL). Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted (IB) with either anti-SU or anti-BiP antibodies. The envelope protein is synthesized in the ER as a polyprotein pr85env (asterisks), which after transit to the Golgi compartment is cleaved by a cellular protease into SU (dot) and TM (not shown) components (see top Fig. 2). The smaller molecular sizes of the pr85env and SU proteins of FrCasNC compared to those of F43 are due to two deletions of 4 and 7 amino acid residues in the PRR (see Fig. 2) of CasBrE. The envelope protein of FrCasNC but not F43 is retained in the ER, which accounts for the difference in the ratio of pr85env and SU proteins observed in F43 and FrCasNC-infected cells (IP-SU/IB-SU). The increased binding of BiP (arrowheads) to the pr85env protein of FrCasNC relative to that of F43 (IP-SU/IB-BiP and IP-BiP/IB-SU) is consistent with the folding instability of the FrCasNC envelope protein reported previously (9).
