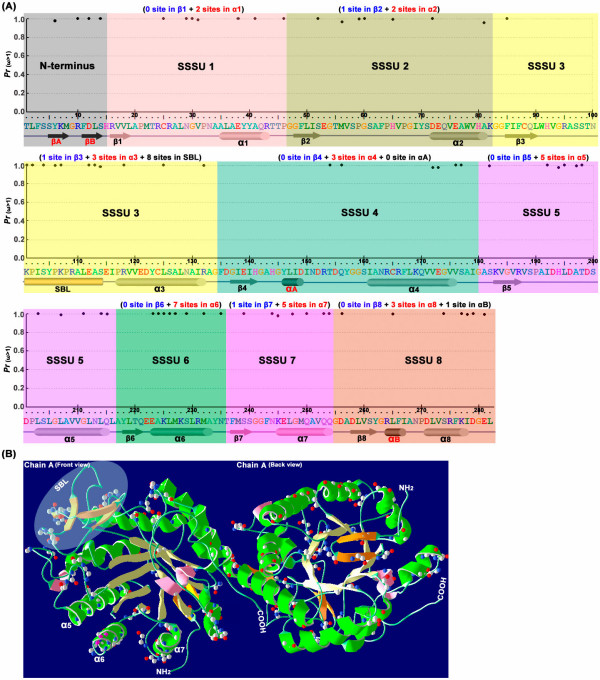
Figure 5.
Distribution of positive-selection sites in the Oxidored_FMN domain. (A) Posterior probabilities for site classes with positive-selection pressures (measured by the ω ratio) for amino acid sites along the sequence. The OPR sequence of the Oxidored_FMN domain is shown below the x-axis along with its secondary structure elements corresponding to the structure of AtOPR1/3 [64,65] and LeOPR3 [66]. Eight α/β-barrel domains are represented as colored tubes and arrows, respectively, and each α/β-barrel is defined as one super secondary structure unit (SSSU). Two β-sheets that form a short hairpin loop at the N-terminus are represented as black arrows. Helix αA, located in SSSU4, and helix αB, part of SSSU8, contribute to a common phosphate-binding motif. And the substrate binding loop (SBL), part of SSSU3, forms the ceiling of the substrate-binding pocket. M3 (discrete) is applied to the data in Table 2. (B) OPR crystal structure from AtOPR3 (PDB ID 1Q45). The crystal structure of AtOPR3, with the backbone shown as a ribbon, was obtained using the Swiss-PdbViewer v4.0 [67]; only chain A is shown, in different orientations: front view (Left) and back view (Right). The 8 α-helices and β-strands in (A) are showed in green and light yellow respectively. Helices αA and αB are shown in pink, and other strands in the N-terminus and SBL are shown in orange. The substrate binding loop (SBL), which contains four β-strands in AtOPR3, is shown in the shaded circle. The positive-selection sites are shown using the as ball-and-stick model, based on the discrete (M3) model (see Table 2).