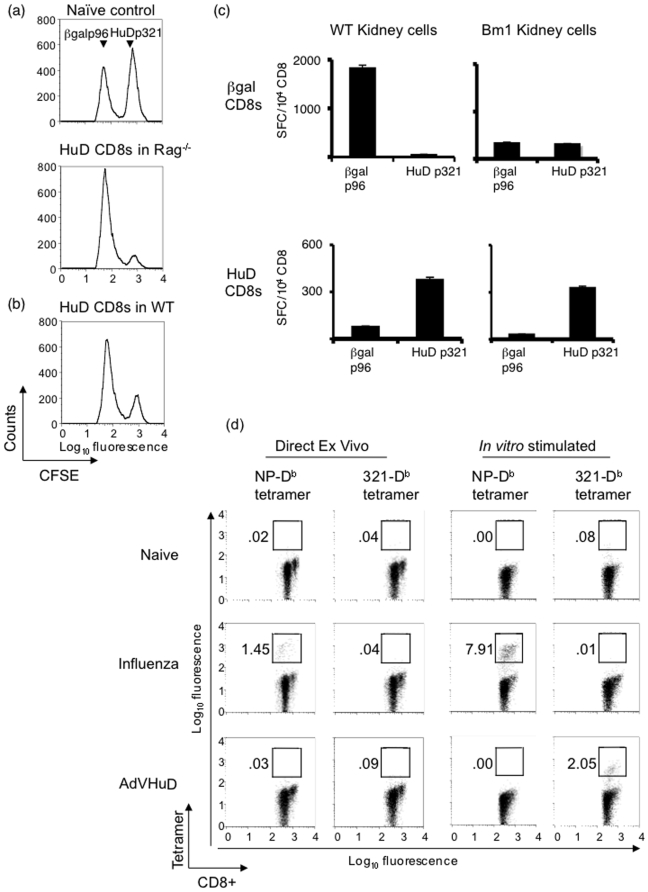
Figure 2. Characterization of HuD p321-specific CD8+ T.
(a) 5×106 HuD p321-specific in vitro stimulated CD8+ T cells were adoptively transferred into Rag−/− mice (n = 2) with 2×106 C57BL/6 DC pulsed with p321. Mice also received PTx and IL-2. Eight days post transfer, mice were injected with CFSE-labeled syngeneic splenocytes pulsed with HuD p321 (CFSEhi) or βgal p96 (CFSElo). A naïve control mouse without transferred CD8+ T cells was injected with CFSE-labeled splenocytes. 6 hours after target injection, splenocytes were analyzed by FACS for in vivo target cell lysis. A representative mouse is shown. Data is representative of two experiments. (b) C57BL/6 mice (n = 2) were used as recipients of adoptively transferred HuD p321-specific CD8+ T cells as in (a). A representative mouse is shown. Data is representative of two experiments. (c) Primary kidney cells from C57BL/6 mice (Db+/Kb+) or transgenic Bm1 mice (Db+/Kb−) were irradiated and pulsed with HuD p321 or βgal p96 and used as stimulators in an IFNγ ELIPOST assay (5×104/well) with 3× restimulated HuD p321-specific or βgal p96-specific CD8+ T cells (104/well). The assay was performed in triplicate. Means are plotted and error bars represent standard deviations of the mean. Data is representative of two experiments. (d) C57BL/6 mice were immunized with either AdVHuD or influenza virus or left untreated (2 mice per group). 15 days after immunization, CD8+ T cells were isolated from the spleen and stained directly ex vivo with anti-CD8+ antibody and PE-labeled tetramer. A portion of splenocytes from each mouse was stimulated in vitro with cognate peptide for 7 days. Naïve mice were stimulated with HuD p321. CD8+ T cells from in vitro stimulation cultures were stained with anti-CD8+ antibody and PE-labeled tetramer. Plots are gated on CD8+ T cells. Data is representative of two experiments.