Short abstract
Brain astrocytes regulate local blood flow and neuronal energy supply by modulating blood vessel tone in response to changes in oxygen levels.
Abstract
Astrocytes mediate either constriction or dilation of local brain arterioles in response to synaptic activity. Recent work indicates that the directionality of this response may be dictated by ambient oxygen levels.
In the brain, astrocyte processes are arranged in coordinated non-overlapping spatial domains such that the vast majority of the surface area of cerebral arterioles and capillaries is contacted by astrocyte endfeet [1]. This makes astrocytes uniquely well positioned to send vasoactive signals to the blood-brain barrier. Several studies now present a consensus view that astrocytes respond to input from glutamate-producing (glutamatergic) neurons by increasing intracellular Ca2+, phospholipase A2 (PLA2)-mediated arachidonic acid formation, and metabolism of arachidonic acid to produce either vasodilatory [2-6] or vasoconstrictor [5,7] metabolites. The conditions under which astrocytes elicit vasodilation or vasoconstriction have remained largely confusing, as either domination of one effect over the other [2,4,7,8] or both effects have been reported in various tissue slice models [5]. In addition, it has not been clear how astrocyte participation in functional or hypoxic hyperemia (an increase in blood flow) fits with the involvement of metabolic mediators such as adenosine, lactate, H+, K+ and CO2, all of which have been implicated in this critical neurophysiological process [9,10]. A recent report from the laboratory of Brian MacVicar (Gordon et al. [11]) significantly clarifies both these issues. Here we discuss this work and give an overview of the multiple pathways by which astrocytes influence neuronal energy supply.
Do astrocytes cause vasodilation or vasoconstriction?
Zonta et al. [2] provided the first direct demonstration that astrocytes in brain slices sense increased levels of glutamate (due to neuronal activity) via metabotropic glutamate receptors (mGluRs) and respond with an increase in endfoot Ca2+, activation of PLA2 and metabolism of arachidonic acid by COX-1 to produce vasodilatory prostaglandins (probably PGE2) [2,12]. Subsequent studies confirmed a vasoactive role for astrocyte mGluR activation and Ca2+ elevation, but reported vasoconstriction rather than vasodilation [7]; constriction was attributed to PLA2-mediated arachidonic acid production by astrocytes, followed by diffusion of the arachidonic acid to smooth muscle and subsequent metabolism by cytochrome P450 4A (ω-hydroxylase) to 20-hydroxyeicosatetraenoic acid (20-HETE). The clear objective left in the wake of these initial papers was to determine whether constriction or dilation was the 'physiological' response to astrocyte activation. Dilation required the presence of a nitric oxide synthase (NOS) inhibitor or other pre-constrictor [2,5-7], and was converted to constriction when NOS inhibitors were excluded [7], raising the possibility that dilations could only be produced by artificial enhancement of baseline vascular tone. On the other hand, constriction was intuitively less attractive as the natural response to increased neuronal work. More recent in vivo studies in anesthetized mice provided strong support for dilation by showing that increased astrocyte Ca2+ levels produced only COX-1-dependent vasodilations and corresponding local increases in blood flow [3]. This was a crucial observation as it verified astrocyte-mediated control of cerebral microcirculation - specifically dilations - outside the brain slice model, which can be critiqued as non-physiological due to the lack of arterial pressurization. It was also shown recently that inhibition of 20-HETE synthesis failed to affect integrated somatosensory hyperemic vasodilation [13], providing further evidence against a physiological role for constrictions.
This dominant vasodilatory effect of astrocyte Ca2+ signaling in vivo called into question the practical significance of the astrocyte- and 20-HETE-mediated constrictions observed in brain slices. Some clarification came from studies showing that both vasodilation and vasoconstriction are possible, depending on the conditions employed [5,14]. One such study found that the polarity of astrocyte vasomotor influence is dependent on pre-existing smooth-muscle tone [14]. In isolated retinas, vasodilations dependent on astrocyte metabolism of arachidonic acid by cytochrome (CYP) P450 2C11 (epoxygenase) to epoxyeicosatrienoic acids (EETs) became less likely as NO levels increased and directly inhibited epoxygenase activity [5]. This identified NO as a pro-constriction factor despite its well-known actions as a direct cGMP-dependent vasodilator [5,15]. These results could explain discrepancies between earlier observations of vasodilations, in the presence of a NOS inhibitor [2], and vasoconstrictions, in conditions of no pre-constriction that probably included higher endogenous NO levels [7]. An opposite pro-dilatory effect of NO has also been observed owing to NO-mediated inhibition of CYP P450 4A and 20-HETE production [13] and it is not yet clear how these effects of NO at CYP P450s are regulated.
The report by Gordon et al. [11] significantly improves our understanding of the balance between vasoconstriction and vasodilation in brain slices. They discovered that although the mGluR-induced rise in astrocyte Ca2+ led to 20-HETE-dependent vasoconstriction in slices maintained in high O2(95%), the same treatment yielded PGE2-dependent vasodilation in slightly hypoxic medium (20% O2). The mechanism for this functional switch is twofold: first, mGluR activation increased the rate of glycolysis in astrocytes, leading to increased extracellular lactate levels sufficient to inhibit PGE2 reuptake via the PGE2/lactate exchanger (PGT). This resulted in a net increase in extracellular PGE2 and a shift in the vasomotor balance from vasoconstriction towards vasodilation. Second, the authors postulate that the final tipping point from dilation to constriction is the action of adenosine at smooth muscle A2A receptors, which overrides the 20-HETE-mediated constrictor effect. They showed that adenosine is produced in low O2, that exogenous adenosine can block 20-HETE-induced vasoconstriction at high (95%) O2, and that mGluR-mediated vasoconstrictions can be converted to dilations by adding exogenous adenosine and PGE2.
Overall, the findings of Gordon et al. [11] effectively establish that astrocytes mediate bidirectional control of local arteriolar diameter in a manner dictated by tissue metabolic status. In this light, one can envisage a 'see-saw'-like balance between vasodilation and vasoconstriction with weight for vasoconstriction provided by 20-HETE and intracellular astrocyte NO, and weight on the vasodilation end provided by adenosine, lactate and PGE2 and/or EETs. It remains to be determined what fulcrum O2 level must be reached before vasodilation is preferred. It is fairly certain that this level is in the normoxic O2 range as in vivo functional hyperemia paradigms at normal PO2 invariably produce dilation and increased cerebral blood flow [3], and the current findings by Gordon et al. [11] show that vasodilation dominated near the low end of physiological PO2 in brain slices. Determination of where the switch point for dilation is may rest with characterizing how low PO2 must fall before ATP production is compromised and adenosine accumulates. While Gordon et al. [11] demonstrated adenosine production in the low physiological range of PO2, it seems unlikely that slice PO2 in the mid to high normoxic range (30-50 mmHg) would produce enough adenosine to maintain dilatory efficacy. At this point, one might predict that vasoconstriction would begin to prevail. Alternatively, it remains possible that local transient increases in extracellular adenosine resulting from breakdown of extracellular ATP during neuro- or gliotransmission could be sufficient to occupy A2A receptors and drive vasodilation independent of a direct effect of PO2.
New ideas about 'old' vasodilators
The paper by Gordon et al. [11] provides valuable hints about how metabolic intermediates, including lactate, H+, K+ and adenosine [9,16,17], might participate in a more regulated and coordinated hyperemic response than would be allowed simply by the diffusion of accumulated metabolites. The authors show that lactate and adenosine, in particular, participate in shifting the astrocyte vasomotor balance from constriction to dilation.
Lactate has direct vasodilatory effects in vitro [17] and augments increases in cerebral blood flow dependent on neuronal activity [10], but Gordon et al. [11] have identified a completely novel mechanism by which it can participate in vasodilation. They confirm previous observations [18] that mGluR activation drives astrocyte glycolysis and produces lactate in low O2, and also show that preventing the conversion of pyruvate to lactate eliminated mGluR-mediated vasodilation in brain slices, making the first specific link between astrocyte lactate and vasodilation. More importantly, they showed that the dilatory effects of lactate are mediated indirectly by PGE2, which is enhanced when extracellular lactate interferes with PGT-mediated exchange of intracellular lactate for extracellular PGE2. These findings establish lactate as a novel operator of the astrocyte vasomotor switch and are consistent with the ability of lactate to augment neuronal-activity-induced increases in blood flow (modeled by Gordon et al. by using mGluR activation) without affecting resting blood flow [10].
It should be noted that while the authors provide evidence that mGluR-driven glycolysis in astrocytes is a source of lactate that can inhibit PGT and enhance extracellular PGE2, the contribution of astrocyte lactate produced by other mechanisms may also be significant. For example, astrocyte lactate can be derived from intracellular Na+ accumulated during glutamate uptake [19]. A significant portion of the hyperemic response in olfactory bulb was recently shown to be dependent on glutamate transport, suggesting that this pathway may represent an important source of lactate [4]. Glycogen mobilization also drives astrocyte lactate production [20] but the contribution of this pathway is not known.
Adenosine acts as an inhibitory neuromodulator in the central nervous system and is implicated in regulating cerebral arterial tone in periods of increased neuronal activity [21] and in hyperemia precipitated by hypoxia [22] and hypoglycaemia [23]. There is also strong evidence for a direct dilatory effect of adenosine at vascular A2A receptors during hyperemia [24]. Gordon et al. [11] showed that an A2A receptor agonist blocked the mGluR-induced vasoconstriction normally observed at 95% O2. They also converted constrictions to dilations in the same high O2 conditions by combining exogenous adenosine with a PGT blocker, which mimicked the increased PGE2 levels observed during hypoxic lactate production at lower O2 levels. These observations support previous reports that A2A receptors are vasodilatory in hyperemia and indicate that multiple vasomotor effects are additive; in other words, A2A-mediated dilation is capable of canceling 20-HETE-induced vasoconstriction.
The experiments of Gordon et al. [11] raise at least three intriguing questions on the role of adenosine in hyperemia. First, the authors nicely demonstrate that exogenous adenosine can compete with 20-HETE-mediated vasoconstriction in conditions of high O2 but do not test whether endogenous adenosine is required to yield dilations at 20% O2. It would be interesting to investigate whether mGluR-mediated vasodilation at 20% O2 is sensitive to inhibition by blocking the effects of endogenous adenosine with an A2A receptor antagonist or exogenous adenosine deaminase. The current evidence leaves open the possibility that accumulation of PGE2 by lactate-mediated inhibition of PGT is sufficient to overcome the effects of 20-HETE and produce dilation without endogenous adenosine. Second, while Gordon et al. [11] undoubtedly show that A2A receptor effects can add to the vascular effects of 20-HETE and PGE2, leading to dilation, it remains to be seen whether other metabolic vasodilators can contribute in the same additive way. It is not yet known, for example, whether H+, K+ or the direct vasodilatory effects of lactate itself can or do compete with the effects of 20-HETE. Lastly, Gordon et al. [11] confirm the widely held consensus that A2A adenosine receptors are likely to play an important role in hyperemia. Given the revelation that astrocyte A2B adenosine receptors regulate EET production and neurovascular coupling in vivo [25], it will be important to dissect the effects of endogenous adenosine to determine whether adenosine acts both at vascular A2A and astrocyte A2B receptors to influence the vasomotor balance toward dilation.
The contribution of Gordon et al. [11] together with that of Metea and Newman [5] show that previously identified vasoactive mediators such as NO, adenosine and lactate may converge on a central control point by influencing an astrocyte-controlled vasomotor balance. The result is a clearer view of the interplay among vasoactive effectors and a conceptually tantalizing model of coordinated spatial cerebral blood flow regulation by targeted vasodilations and vasoconstrictions mediated by Ca2+ signal propagation in distinct astrocyte networks.
Astrocytes coordinate a multimodal nutritive response to challenged neurons
The findings that astrocyte vasomotor polarity depends on oxygen-influenced changes in adenosine and activity-driven extracellular lactate concentrations are novel and exciting but do not exist in isolation. Rather, they are part of a multifaceted response by astrocytes to neuronal energy demand that also involves direct shuttling of tricarboxylic acid (TCA) cycle carbon sources from astrocytes to neurons (Figure 1). Perivascular astrocytes react in several ways to glutamatergic signals from neurons. Glutamate released by active neurons is rapidly removed from the synapse by high-affinity astrocyte glutamate transporters [26] and the internalized glutamate is converted to glutamine, which is released by astrocytes and claimed by neurons. This enables neurons to avoid a net loss of glutamate during neurotransmission and provides a potential TCA substrate for synthesis of γ-aminobutyric acid and production of ATP. Lactate is also shuttled from astrocytes to neurons for use as an oxidative fuel. Lactate increases have been reported in response to mGluR activation [18] and Na+-dependent glutamate transporter activity [19], and lactate can also be derived from mobilization of astrocyte glycogen in response to neuronal activity [20,27]. Intracellular lactate is released from astrocytes via the monocarboxylate transporter-1 (MCT1) where it can be taken up by neuronal MCT2 and converted to pyruvate for use in the TCA cycle. Importantly, astrocyteneuron metabolic communication, and probably vasomotor communication, cannot be viewed simply as signaling between discrete cell pairs or even among small groups of cells. New information affirms that lactate from cerebral arterioles is delivered over large distances along gap-junction-coupled astrocyte networks to areas of stimulated neurons but not to resting neurons [28]. Overall, astrocytes mount a multipronged neuronal aid effort centered largely on the multiple vasomotor and metabolic effects of lactate.
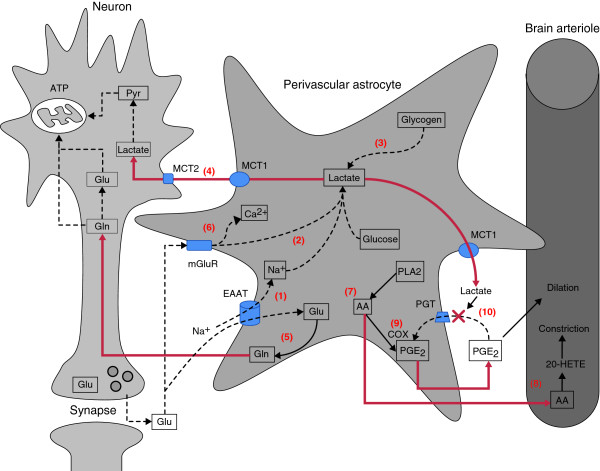
Figure 1.
Astrocyte influences on neuronal energy supply. Perivascular astrocytes respond to neuronal input (activity) by supplying neurons with substrates for oxidative phosphorylation (lactate, glutamine (Gln)) and glutamate (Glu) replenishment (glutamine), and by signaling changes in local blood flow at the vascular level. Active neurons produce synaptic glutamate that can be taken up by astrocyte glutamate transporters (EAAT) or activate mGluRs. (1) EAAT activation drives electrogenic Na+ influx, activates Na+/K+ ATPases and stimulates glycolytic lactate generation. (2) mGluR activation also leads to glycolysis and lactate production, and neuronal activity drives astrocyte glycogenolysis (3) and eventual lactate formation. Lactate from these three sources is released to the extracellular space via monocarboxylate transporter 1 (MCT1) where it can be taken up by neuronal MCT2 and converted to pyruvate (Pyr) for entry into the TCA cycle (4). Glutamate taken up by astrocyte EAATs can also be converted to glutamine by glutamine synthetase (5). Glutamine can be released and taken up by neuronal amino acid transporters for re-synthesis of glutamate and/or γ-aminobutyric acid via the TCA cycle. For astrocyte changes in blood flow, mGluR activation causes increased Ca2+ levels (6), leading to phospholipase A2 (PLA2) activation, arachidonic acid (AA) formation (7) and vasoconstriction following 20-HETE production by cytochrome P450 ω-hydroxylase (8) and continuous prostaglandin E2 (PGE2) generation by cyclooxygenase (COX) (9). Vasodilation can result in hypoxic conditions from lactate-mediated inhibition of PGE2 clearance by prostaglandin transporters (PGT) following PGE2 diffusion to the vascular smooth muscle (10). EAAT, excitatory amino acid transporter; Pyr, pyruvate.
Studies over the past five years have revealed that astrocytes link neuronal energy supply and demand by triggering adaptive changes in the delivery of blood-borne glucose and O2 to neurons. Gordon et al. [11] have taken the O2 understanding of this to a new level by showing that astrocytes can act as switches to either increase or decrease blood flow to working neurons depending on regional metabolic status. Their study will serve as an important launch point for future work aimed at identifying how astrocyte networks regulate the spatial control of brain blood flow both near to and distant from areas of neuronal activation.
Acknowledgments
Acknowledgements
We thank the Canadian Institute of Health Research and Manitoba Health Research Council for research support. CMA is supported by the Heart and Stroke Foundation of Canada. JLM is supported by a doctoral research award from the Canadian Institutes of Health Research.
References
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Edvinsson L, MacKenzie ET. The concept of coupling blood flow to brain function: revision required? Ann Neurol. 1987;22:289–297. doi: 10.1002/ana.410220302. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Vlassenko AG, Rundle MM, Raichle ME. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci USA. 2004;101:659–664. doi: 10.1073/pnas.0307457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295:H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I. Cytochrome P450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-G. [DOI] [PubMed] [Google Scholar]
- Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-Z. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Meno JR, Hochman DW, Ngai AC, Winn HR. Correlation of intrinsic optical signal, cerebral blood flow, and evoked potentials during activation of rat somatosensory cortex. J Neurosurg. 2008;109:654–663. doi: 10.3171/JNS/2008/109/10/0654. [DOI] [PubMed] [Google Scholar]
- Morii S, Ngai AC, Ko KR, Winn HR. Role of adenosine in regulation of cerebral blood flow: effects of theophylline during normoxia and hypoxia. Am J Physiol. 1987;253:H165–H175. doi: 10.1152/ajpheart.1987.253.1.H165. [DOI] [PubMed] [Google Scholar]
- Ruth VJ, Park TS, Gonzales ER, Gidday JM. Adenosine and cerebrovascular hyperemia during insulin-induced hypoglycemia in newborn piglet. Am J Physiol. 1993;265:H1762–H1768. doi: 10.1152/ajpheart.1993.265.5.H1762. [DOI] [PubMed] [Google Scholar]
- Miekisiak G, Kulik T, Kusano Y, Kung D, Chen JF, Winn HR. Cerebral blood flow response in adenosine 2a receptor knockout mice during transient hypoxic hypoxia. J Cereb Blood Flow Metab. 2008;28:1656–1664. doi: 10.1038/jcbfm.2008.57. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. doi: 10.1002/1098-1136(200010)32:1<1::AID-GLIA10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]