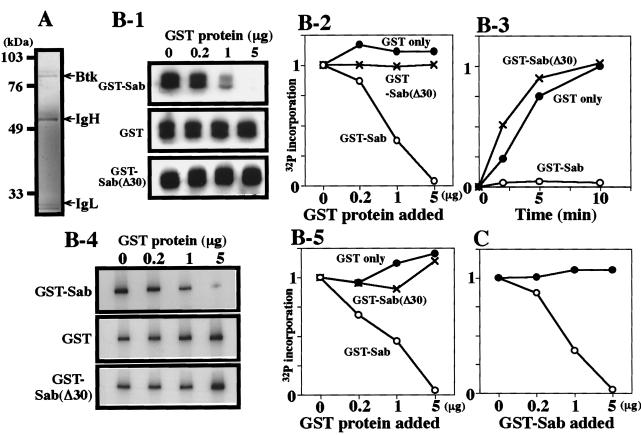
Figure 2.
(A) Silver staining of the immunopurified Btk protein used in this experiment. IgH represents the Ig heavy chain, and IgL represents the light chain used for immunopurification of the Btk protein. Molecular mass standards are shown in kDa. (B-1 and B-2) Dose-dependent inhibition of the transphosphorylation activity of Btk by Sab. Approximately 10 ng of the immunopurified Btk protein was mixed with the indicated amounts of GST (●), GST-Sab (○), or GST-Sab (Δ30) (×) protein, and in vitro kinase reactions were performed with the addition of the peptide substrate. The relative radioactive incorporation into the peptide substrate is shown. (B-3) Time course of radioactive incorporation into the peptide substrate. In vitro Btk kinase reaction was performed with the peptide substrate in the presence of 5 μg of GST proteins. (B-4 and B-5) Dose-dependent inhibition of the Btk autophosphorylation by Sab. The conditions for the Btk kinase reaction were the same as those for B-1 and B-2. (C) Phosphatase activity is not responsible for the observed reduction of the Btk kinase activity seen in B-1 and B-2. The in vitro kinase reaction was performed under the same conditions as those for B-1 and B-2 but without the GST protein. After the reaction was terminated, the mixture was incubated with the indicated amounts of the GST-Sab protein for another 10 min at 25°C. Radioactive incorporations were measured by scintillation counting of the phosphorylated peptides (●). For comparison, the open circles show the reduction in the radioactive incorporation into the peptide substrate as a result of the addition of the GST-Sab protein before the in vitro kinase reaction (the same dose-dependent reduction as shown in B-2).