Abstract
Background
Previous studies demonstrate impairment of endothelial-dependent vasodilation after ischemia/reperfusion (I/R). Though we have demonstrated that inhibition of δ protein kinase C (δPKC) at reperfusion reduces myocyte damage and improves cardiac function in a porcine acute myocardial infarction (AMI) model, impact of the selective δPKC inhibitor on epicardial coronary endothelial function remains unknown.
Methods
Either δPKC inhibitor (δV1-1, n=5) or saline (n=5) was infused into the left anterior descending artery at the last 1 minute of the 30-minute ischemia by balloon occlusion. In vivo responses to bradykinin (endothelium-dependent vasodilator) or nitroglycerin (endothelium-independent vasodilator) were analyzed at 24 h after I/R using intravascular ultrasound. Vascular responses were calculated as the ratio of vessel area at each time point (30, 60, 90 and 120 seconds after the infusion), divided by values at baseline (before the infusion).
Results
In control pigs, endothelial-dependent vasodilation following bradykinin infusion in infarct-related epicardial coronary artery was impaired, whereas in δPKC inhibitor treated-pigs the endothelial-dependent vasodilation was preserved. Nitroglycerin infusion caused similar vasodilatory responses in the both groups.
Conclusions
This is the first demonstration that a δPKC inhibitor preserves vasodilator capacity in epicardial coronary arteries in an in vivo porcine AMI model. Because endothelial dysfunction correlates with worse outcome in patients with AMI, this preserved endothelial function in epicardial coronary arteries might result in a better clinical outcome.
Keywords: ultrasonography, angioplasty, myocardial infarction, protein kinases, endothelium
1. Introduction
Despite improved outcomes with early coronary artery reperfusion for the treatment of acute ST-elevation myocardial infarction, morbidity and mortality from acute myocardial infarction (AMI) remain significant (1). Even following successful recanalization of the occluded artery, AMI patients demonstrate impaired microvascular flow during reperfusion, leading to increased myocardial infarct size (2–5). We have recently demonstrated that administration of a selective δ protein kinase C (δPKC) peptide inhibitor, δV1-1 (6), just before reperfusion, reduces reperfusion injury-induced myocyte damage in a porcine AMI model (7,8).
Previous animal and human studies have suggested that the epicardial coronary artery (large vessel) in the infarcted area demonstrates impairment endothelial-dependent vasodilation ability (endothelial dysfunction) (9,10), and this endothelial dysfunction correlates with poor outcomes in AMI patients. Therefore, in this study, we examine the impact of δV1-1, the δPKC inhibitor, on the vasodilator capacity of epicardial coronary arteries in the infarcted area using intravascular ultrasound (IVUS) in an in vivo porcine AMI model.
2. Materials and Methods
2.1. Animals
All animal studies were approved by Stanford’s Institutional Animal Care and Use Committee.
Yorkshire swine (30–45kg) were maintained under anesthesia by inhaled isoflurane (1–2%). A bolus of 300 IU/kg heparin was administered intravenously through the sheath (6 French) placed in the carotid artery. A 10 mm over-the-wire angioplasty balloon was placed in the left anterior descending artery (LAD) proximal to the first diagonal branch. The balloon was inflated to occlude the LAD for 30 minutes. At the last 1 minute of the 30-minute ischemia, Tat-conjugated δV1-1(6) (250 ng/kg) or saline was infused at 1 mL/minute through the guide-wire lumen of the balloon catheter (n=5 for each group), as previously described (7,8).
2.2. Intravascular Ultrasound Imaging
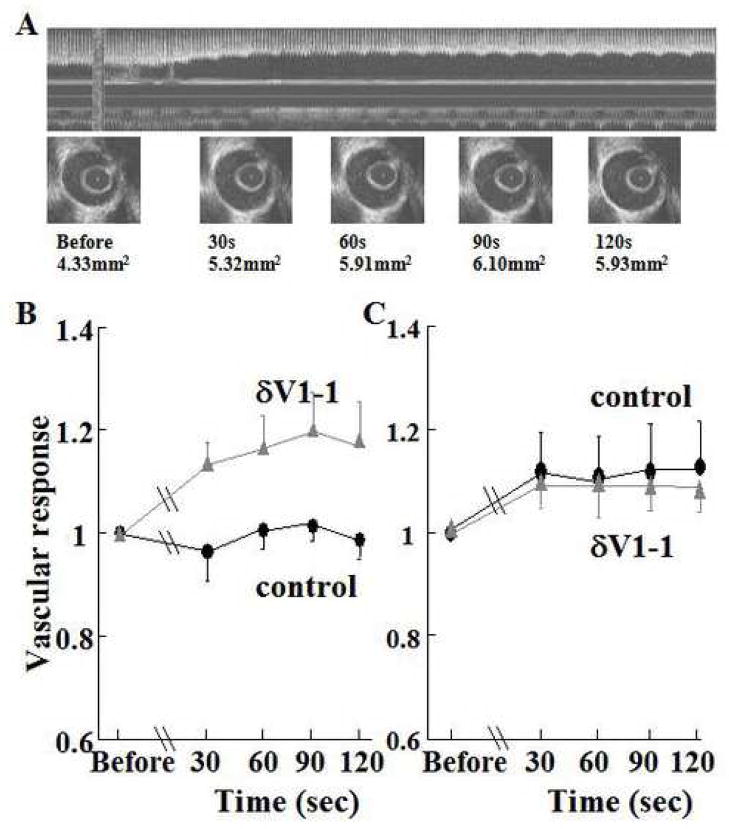
In vivo responses of large vessels to bradykinin (endothelium-dependent vasodilator) or nitroglycerin (endothelium-independent vasodilator) were analyzed at 24 hours after reperfusion using intravascular ultrasound (IVUS) (11,12). Here, we evaluated the vasodilator response to bradykinin. We did not use acetylcholine, since the response of porcine coronary artery to acetylcholine is vasospastic, rather than vasodilatory even in the absence of ischemia (13). A 2.9 Fr 40-MHz IVUS catheter (Boston Scientific, Natick, MA) was passed over the wire and positioned in the LAD distal to the previously occluded segment. Bradykinin (0.2ml of a 3×10−6M solution in saline) was infused into the LAD through the guide catheter for 10 seconds.(14) IVUS data were acquired from 10 seconds before the infusion and for 120 seconds thereafter. Five minutes later, nitroglycerin (0.2mg in saline) was also infused into the LAD of the same animal, and IVUS data acquired in a similar manner. All measurements were performed by a blinded observer with the use of a computer-based imaging system (echoPlaque, Indec Systems, Inc, Mountain View, CA). Lumen areas were manually traced before the infusion (baseline), and at 30, 60, 90 and 120 seconds after the infusion (Figure 1A). At each time point, three serial images were obtained at end-diastole and those values were averaged. Vascular responses were calculated as the ratio of averaged lumen area at each time point divided by values at baseline.
Figure 1.
(A) Intravascular ultrasound (IVUS) imaging of coronary artery after bradykinin infusion
Upper panel shows reconstructed longitudinal image (echoPlaque, Indec Systems, Inc, Mountain View, CA). Lower panels show cross-sectional images of coronary artery at each time point. Lumen areas at each time point are shown.
(B) δPKC inhibition by δV1-1 preserves endothelial-dependent vasodilation (stimulated by bradykinin) from ischemia and reperfusion-induced injury
In control pigs, endothelial-dependent vasodilator capacity following bradykinin infusion in infarct-related epicardial coronary artery was measured after treatemnet with δV1-1 or Tat control. Vascular responses were calculated as the ratio of averaged lumen area at each time point, divided by values at baseline. Data are expressed as mean plus or minus standard error of the mean. In δV1-1-treated pigs, the endothelial-dependent vasodilator capacity was preserved (P=0.036, δV1-1-treated (n=5) vs. control (n=5); treatment-time interaction P=0.010).
(C) Endothelial-independent vasodilatation (stimulated by nitroglycerin) is not affected by treatment with δV1-1
Nitroglycerin was infused into the arteries and vasodilatation measured. Nitroglycerin-induced vasodilatory responses in both groups were similar (P=0.81, δV1-1-treated (n=4) vs. control (n=5); treatment-time interaction P=0.98).
2.3. Data analysis
Data are expressed as mean ± standard error of the mean. Two-way analysis of variance for repeated measures was used for the time-course of vascular responses. P<0.05 was considered statistically significant.
3. Results and Discussion
To evaluate the effects of δV1-1 treatment in endothelial-dependent vasodilator capacity, lumen area change after bradykinin infusion in the δV1-1-treated group was compared to that of the control group using IVUS analysis. In control pigs, endothelial-dependent vasodilator capacity following bradykinin infusion in infarct-related epicardial coronary arteries was impaired, while in δV1-1-treated pigs, the endothelial-dependent vasodilator capacity was preserved (Figure 1B). Nitroglycerin infusion caused similar vasodilatory responses in both groups, suggesting preserved endothelial-independent vasodilator capacity (Figure 1C).
We have recently demonstrated that administration of a selective δPKC peptide inhibitor, δV1-1, just before reperfusion reduces myocardial infarct size in a porcine model of AMI.(7) Here, we demonstrate that this δPKC inhibitor preserved the vasodilator capacity in epicardial coronary arteries in this in vivo porcine model. Because endothelial dysfunction is related to worsened outcomes in AMI patients, this preserved endothelial function may be, in part, responsible for improved clinical outcomes for these patients.
Our data are consistent with other recent studies, which assess endothelial dysfunction after ischemia and reperfusion. In one study, both coronary flow reserve and electron microscopy in a porcine AMI model demonstrated improved microvascular function due to maintenance of open microvessel following δV1-1 treatment (7,8). In a second study of middle cerebral artery occlusion, δV1-1 treatment resulted in improved microvascular pathology, a 92% increase in number of patent microvessels and a 26% increase in cerebral blood flow following acute focal ischemia (15). Together with our study here, examining endothelial cell function in coronary arteries using IVUS, a picture emerges suggesting that increased cardiac injury following ischemia may be due, at last in part, to endothelial cell dysfunction, and that dysfunction can be reduced by inhibiting δPKC.
In 1982, Ku et al.(9) demonstrated the first evidence of coronary endothelial dysfunction after ischemia and reperfusion, as evidenced by an impaired endothelium-dependent vasodilatation. Endothelial dysfunction occurs early during reperfusion of previously ischemic tissue and may present for an extended time period (4 to 12 weeks) (16–18). Endothelial dysfunction facilitates the expression of a pro-thrombotic phenotype, characterized by platelet and neutrophil activation. Even after successful thrombolysis, 7% of patients suffered from re-infarction in recent fibrinolytic trials, which tends to be associated with poor clinical outcomes (19). Although catheter-based mechanical reperfusion strategies result in improved clinical outcome over pharmacological reperfusion strategies (20), fatal or non-fatal myocardial (re)infarction still occurs with more frequency in AMI patients than in patients with stable angina (21). Superimposed on the inherent systemic vulnerability (hyper-coagulability, inflammatory state) in AMI patients, endothelial dysfunction in coronary arteries is likely to play a role in the development of further acute coronary events. Therefore, our findings that δPKC inhibition with δV1-1 improves endothelial function may translate to improved patient outcome, if these data are corroborated also in humans.
Endothelial dysfunction may also lead to a reduced production of the endothelium-derived vasodilator, nitric oxide. Since nitric oxide has several anti-atherogenic properties, including inhibition of platelet aggregation and smooth muscle cell proliferation, a dysfunctional endothelium may further contribute to a pro-atherogenic state and plaque instability. In experimental animals, nitric oxide availability is inversely related to disease progression. Thus, post MI endothelial dysfunction, exaggerated by ischemia-reperfusion, may accelerate preexisting atherosclerosis in patients with AMI, leading to future ischemic events. Improved endothelial function by inhibiting δPKC may therefore provide an additional benefit, namely reducing plaque instability.
Although angiography has been the predominant method to define coronary anatomy as well as function in the past 40 years, many studies have challenged the accuracy and reproducibility of this technique (22). Well known are the effects of contrast agents on heart-myocardial contractility, electrophysiology and coronary blood flow, all of which may interfere with vascular responses (23,24). Recently, new and emerging applications for IVUS imaging provide an alternative to angiography. Indeed, continuous in vivo assessment of lumen area by IVUS without contrast agent injection enables a more accurate and detailed analysis of vascular response and health and therefore may better guide therapeutic protocols.
Impact of the δPKC inhibitor on the vasodilator capacity may differ between animals (juvenile but otherwise healthy pig coronary artery) and humans (diseased coronary artery with preexisting plaque rupture, erosion (25) and/or dysfunctional endothelium and systemic co-morbid conditions (diabetes, hypercholesterolemia, hypertension and so on). Further clinical investigation is needed to determine whether this novel cardioprotective therapy might improve clinical outcomes in patients with AMI.
In conclusion, in addition to reducing myocardial infarct size in the acute phase, this novel cardioprotective therapy of a selective δPKC inhibitor may further improve long-term outcome in patients with AMI, through the preservation of endothelial function, thereby improving flow through the coronary arteries and preventing new ischemic events.
Acknowledgments
This work was supported by NIH grant HL-52141 (to Dr Mochly-Rosen).
Footnotes
Disclosure
Dr Mochly-Rosen is a founder of KAI Pharmaceuticals, a pharmaceutical company that aims to bring peptide regulators of PKC to the clinic. However, the research described in this study was carried out in her laboratory at the university, independent of the company and with sole support from the NIH for her university activities.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–86. doi: 10.1016/j.jacc.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 2.Maroko PR, Kjekshus JK, Sobel BE, et al. Factors influencing infarct size following experimental coronary artery occlusions. Circulation. 1971;43:67–82. doi: 10.1161/01.cir.43.1.67. [DOI] [PubMed] [Google Scholar]
- 3.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–9. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reffelmann T, Kloner RA. The “no-reflow” phenomenon: basic science and clinical correlates. Heart. 2002;87:162–8. doi: 10.1136/heart.87.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Hahn H, Wu G, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–9. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inagaki K, Chen L, Ikeno F, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–7. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 8.Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Impaired perfusion after myocardial infarction is due to reperfusion-induced deltaPKC-mediated myocardial damage. Cardiovasc Res. 2007;73:699–709. doi: 10.1016/j.cardiores.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ku DD. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982;218:576–8. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- 10.Okumura K, Yasue H, Matsuyama K, et al. Effect of acetylcholine on the highly stenotic coronary artery: difference between the constrictor response of the infarct-related coronary artery and that of the noninfarct-related artery. J Am Coll Cardiol. 1992;19:752–8. doi: 10.1016/0735-1097(92)90513-m. [DOI] [PubMed] [Google Scholar]
- 11.Sudhir K, MacGregor JS, Barbant SD, et al. Assessment of coronary conductance and resistance vessel reactivity in response to nitroglycerin, ergonovine and adenosine: in vivo studies with simultaneous intravascular two-dimensional and Doppler ultrasound. J Am Coll Cardiol. 1993;21:1261–8. doi: 10.1016/0735-1097(93)90255-y. [DOI] [PubMed] [Google Scholar]
- 12.Kuga T, Egashira K, Mohri M, et al. Bradykinin-induced vasodilation is impaired at the atherosclerotic site but is preserved at the spastic site of human coronary arteries in vivo. Circulation. 1995;92:183–9. doi: 10.1161/01.cir.92.2.183. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Morishita R, Asai T, et al. Molecular strategy using cis-element ‘decoy’ of E2F binding site inhibits neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2002;9:488–94. doi: 10.1038/sj.gt.3301679. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Sinovas A, Bis J, Anivarro I, de la Torre J, Bayes-Genis A, Cinca J. Coronary smooth muscle reactivity to muscarinic stimulation after ischemia-reperfusion in porcine myocardial infarction. J Appl Physiol. 2003;95:81–8. doi: 10.1152/japplphysiol.00119.2003. [DOI] [PubMed] [Google Scholar]
- 15.Bright R, Steinberg GK, Mochly-Rosen D. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 2007;1144:146–55. doi: 10.1016/j.brainres.2007.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res. 1996;32:743–51. [PubMed] [Google Scholar]
- 17.Kaeffer N, Richard V, Francois A, Lallemand F, Henry JP, Thuillez C. Preconditioning prevents chronic reperfusion-induced coronary endothelial dysfunction in rats. Am J Physiol. 1996;271:H842–9. doi: 10.1152/ajpheart.1996.271.3.H842. [DOI] [PubMed] [Google Scholar]
- 18.Pearson PJ, Schaff HV, Vanhoutte PM. Long-term impairment of endothelium-dependent relaxations to aggregating platelets after reperfusion injury in canine coronary arteries. Circulation. 1990;81:1921–7. doi: 10.1161/01.cir.81.6.1921. [DOI] [PubMed] [Google Scholar]
- 19.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 20.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 21.Al Suwaidi J, Holmes DR, Jr, Salam AM, Lennon R, Berger PB. Impact of coronary artery stents on mortality and nonfatal myocardial infarction: meta-analysis of randomized trials comparing a strategy of routine stenting with that of balloon angioplasty. Am Heart J. 2004;147:815–22. doi: 10.1016/j.ahj.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–16. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 23.Dawson P. Cardiovascular effects of contrast agents. Am J Cardiol. 1989;64:2E–9E. doi: 10.1016/0002-9149(89)90727-3. [DOI] [PubMed] [Google Scholar]
- 24.Tatineni S, Kern MJ, Deligonul U, Aguirre F. The effects of ionic and nonionic radiographic contrast media on coronary hyperemia in patients during coronary angiography. Am Heart J. 1992;123:621–7. doi: 10.1016/0002-8703(92)90499-l. [DOI] [PubMed] [Google Scholar]
- 25.Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–63. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]