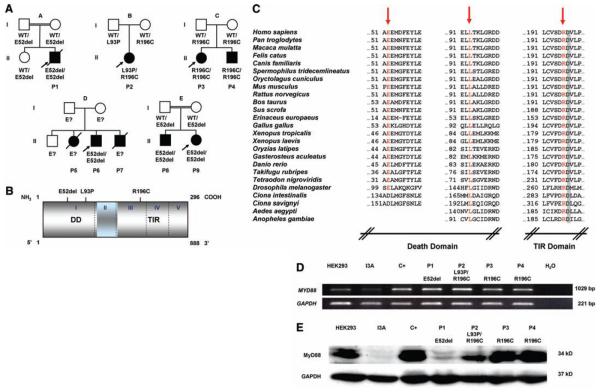
Fig. 1.
(A) Kindreds and patients with MYD88 mutations. (B) Positions of the MyD88 mutations in the death domain (DD) or the TIR domain of the protein. (C) Parts of the DD and TIR domain of MyD88 in humans and the corresponding regions in 24 other species. The residues mutated are indicated in red. Amino acid D197 (gray) is conserved in all species. (D) Full-length MYD88 transcripts in SV40-transformed fibroblasts from a healthy control donor (C+), four MyD88-deficient patients (P1 to P4), the MyD88-deficient HEK293 cell line (I3A), and the parental MyD88-positive HEK293 cell line. (E) MyD88 protein expression in SV40-transformed fibroblasts from a healthy control (C+), four patients (P1 to P4), the I3A line, and the parental HEK293 cell line. The MyD88-specific antibody recognizes residues 279 to 296. Abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr.