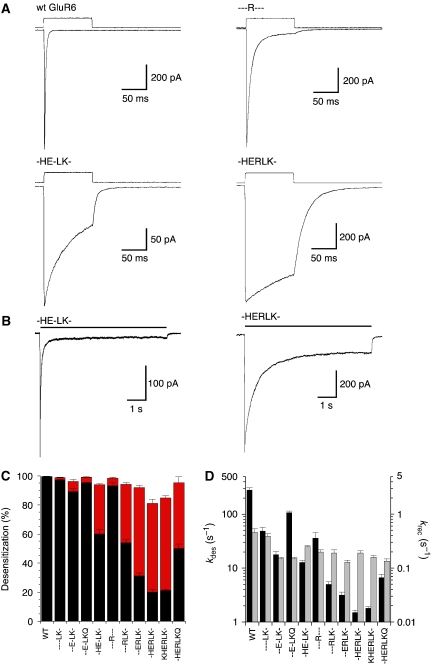
Figure 2.
Kinetic analysis for a library of GluR6 dimer interface mutants. (A) Responses of outside-out patches to 100-ms applications of 10 mM glutamate are shown for wild type and three mutants. The rate of desensitization (kdes) is fastest for wild type, ∼200-fold slower for -HERLK-, whereas - - -R- - - and -HE-LK- produce intermediate kinetics. (B) Responses to 7-s applications of glutamate show the extent of desensitization for -HE-LK- and -HERLK-, highlighting the impact of the K665R mutation in the -HE-LK- background. (C) The extent of desensitization measured at 100 ms (black) and 7 s (red). (D) Rate of onset of desensitization (black bar) and recovery (grey bar), for wild-type GluR6 and the library of 10 dimer interface mutants; error bars indicate mean±s.e.m.