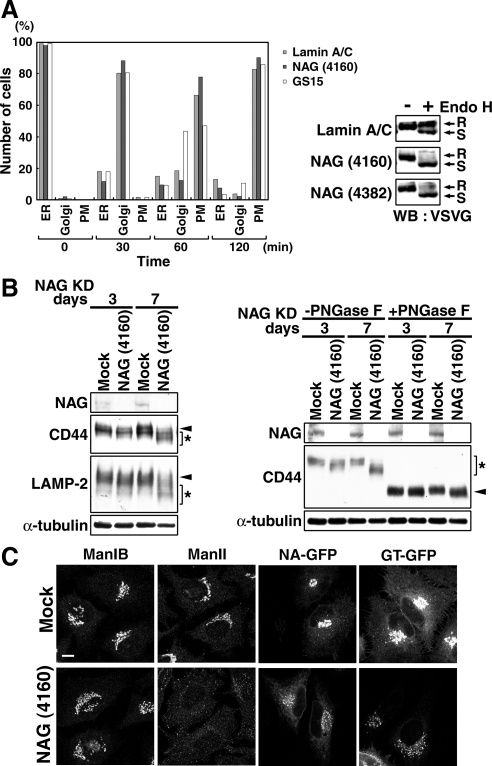
Figure 8.
Depletion of NAG causes a defect in glycosylation of secretory and lysosomal proteins. (A) HeLa cells were successively transfected with lamin A/C siRNA, NAG (4160), or GS15 siRNA and then with the plasmid for VSVG-GFP. Transport of VSVG-GFP was monitored as described in Materials and Methods. The number of cells in which VSVG-GFP was localized in the ER, the Golgi apparatus, and plasma membrane (PM) were counted. Alternatively, lysates of cells double transfected were prepared after 120-min incubation at the permissive temperature and treated with endoglycosidase H (Endo H). The samples were subjected to SDS-PAGE and analyzed by immunoblotting with an anti-VSVG antibody. R, Endo H-resistant bands; S, Endo H-sensitive bands. (B, left) HeLa cells were mock treated or treated with NAG (4160) for 3 or 7 d, solubilized in phosphate-buffered saline with 0.5% SDS, separated by SDS-PAGE, and analyzed by immunoblotting with the indicated antibodies. Arrowheads and asterisks indicate fully glycosylated and underglycosylated proteins, respectively. (B, right) Lysates of HeLa cells depleted of NAG for 3 or 7 d were treated with PNGase F. Samples were loaded on SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Asterisk and arrowheads indicate the positions of glycosylated and deglycosylated forms of CD44, respectively. (C) Distribution of glycosylation enzymes in HeLa cells treated with NAG (4160) for 7 d. In the expression of N-acetylglucosaminyltransferase I-GFP (NA-GFP) and β-1,4-galactosyltransferase 1-GFP (GT-GFP), the plasmids encoding GFP fusion proteins were transfected into HeLa cells at 6 d after transfection with NAG (4160). Bar, 10 μm.