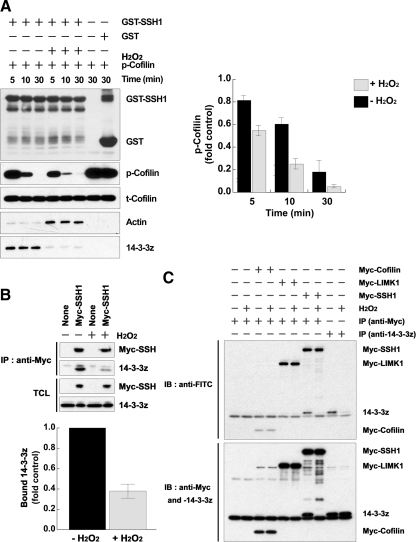
Figure 2.
H2O2 disrupts the complex of SSH-1L and 14-3-3 through 14-3-3 oxidation. (A) HeLa cells were transfected with GST-SSH-1L for 18 h and then treated with H2O2 for 30 min. GST-SSH-1L proteins were precipitated from cell lysates and incubated with purified phosphocofilin for 0–30 min. Cofilin dephosphorylation was measured by immunoblotting with a phosphorylated cofilin antibody (p-Cofilin) and total cofilin, GST-SSH-1L, bound actin, or 14-3-3ζ were detected by immunoblotting with anti-cofilin, -GST, -actin, or -14-3-3ζ antibodies. The graph represents the averaged normalized p-cofilin values; the value with GST alone is taken as 1.0. Data are from three independent experiments ± SD. (B) HeLa cells transfected with empty vector (control) or myc-SSH-1L construct were treated with or without H2O2 for 30 min. The cells were then subject to lysis and immunoprecipitation with anti-myc antibodies. Immunoprecipitated Myc-SSH1L and bound 14-3-3 proteins were detected by immunoblotting with each anti-Myc or -pan-14-3-3 antibodies. The graph below represents averaged normalized bound 14-3-3 values; the value in Myc-SSH-1L–expressing cells treated without H2O2 is taken as 1.0. Data are from three independent experiments ± SD. (C) HeLa cells were transfected with the indicated cDNA for 18 h and then treated with or without H2O2 for 30 min. The cell lysates were prepared and proteins were labeled with 5′-iodoacetamidofluorescein (Wu et al., 1998). The labeled proteins were immunoprecipitated (IP) with anti-Myc or -14-3-3ζ antibodies, followed by immunoblotting (IB) with anti-FITC antibodies. The total immnoprecipitated proteins were analyzed by immunoblotting with anti-Myc or -14-3-3ζ antibodies. Oxidation of the redox-sensitive cysteine group(s) results in the loss of fluorescein labeling and thus the loss of anti-FITC reactivity. Representative experiments from three separate experiments are shown.