Abstract
OBJECTIVE: To review our experience with video-assisted thoracoscopic (VATS) lobectomy with respect to morbidity, mortality, and short-term outcome.
PATIENTS AND METHODS: VATS lobectomies were performed in 56 patients between July 6, 2006, and February 26, 2008. Two patients declined consent for research participation and were excluded. Clinical data for 54 patients were collected from medical records and analyzed retrospectively.
RESULTS: The studied cohort included 19 men (35%) and 35 women (65%) with a median age of 67.5 years (minimum-maximum, 21-87 years; interquartile range [IQR], 59-74 years). Median duration of operation for VATS lobectomy was 139 minutes (minimum-maximum, 78-275 minutes; IQR, 121-182 minutes). Two cases (4%) required conversion to open lobectomy. Median time to chest tube removal was 2 days (minimum-maximum, 1-12 days; IQR, 1.3-3.8 days). Median length of stay was 4 days (minimum-maximum, 1-12 days; IQR, 4-7 days). There was no operative mortality.
CONCLUSION: VATS lobectomy is safe and feasible for pulmonary resection. This minimally invasive approach may allow patients to benefit from lobectomy with shorter recovery times and hospital stays compared with conventional open thoracotomy.
The authors reviewed their experience with video-assisted thoracoscopic lobectomy with respect to morbidity, mortality, and short-term outcome and found that this procedure is safe and feasible for pulmonary resection. This minimally invasive approach may allow patients to benefit from lobectomy with shorter recovery times and hospital stays compared with conventional open thoracotomy.
IQR = interquartile range; NSCLC = non-small cell lung cancer; VATS= video-assisted thoracoscopic
Video-assisted thoracoscopic (VATS) surgery was first described by several groups in the early 1990s. Initial applications included exploration of the chest, management of pleural effusion or pneumothorax, and limited resection of lung nodules.1-6 In subsequent years it has achieved broad application in clinical practice as a minimally invasive tool for multiple indications.7-20 Techniques for VATS lobectomy as an oncologic resection emerged after this experience, with many large retrospective series showing the feasibility and safety of this minimally invasive surgical approach.11,12,14-16,21-23 Although the existing retrospective data suggest equivalent oncologic outcomes with VATS lobectomy and lobectomy using a conventional open thoracotomy, prospective studies are needed to confirm this hypothesis and are ongoing.24 Compared with the open approach, VATS lobectomies have the potential advantage of decreased postoperative pain and a shorter hospital length of stay.25-27 Other proposed advantages of a thoracoscopic approach include decreased blood loss, fewer postoperative complications, preserved pulmonary function, decreased inflammatory response, and a more rapid return to preoperative activity.10,11,14,15,20,25 If adjuvant chemotherapy or radiation therapy is indicated, a potential shorter postoperative recovery time may allow adjuvant treatment at a shorter postoperative interval with better adherence and treatment completion rates.12,28
VATS lobectomies have been used routinely in our department since July 2006. This report reviews our experience with the VATS approach with respect to morbidity and mortality as well as its potential effect on the length of patient hospitalization.
PATIENTS AND METHODS
Patients were identified from a database of operations performed by the Division of General Thoracic Surgery at Mayo Clinic's site in Rochester, MN. Between July 6, 2006, and February 26, 2008, 56 patients were selected to undergo VATS lobectomy and 320 patients to undergo open lobectomy. Our selection criteria for VATS continue to evolve.
The study was approved by the Mayo Clinic Institutional Review Board. Two patients were not included in this study because they declined consent for research participation. The demographic and medical data of the remaining 54 patients were collected from their medical records and analyzed retrospectively.
Procedure
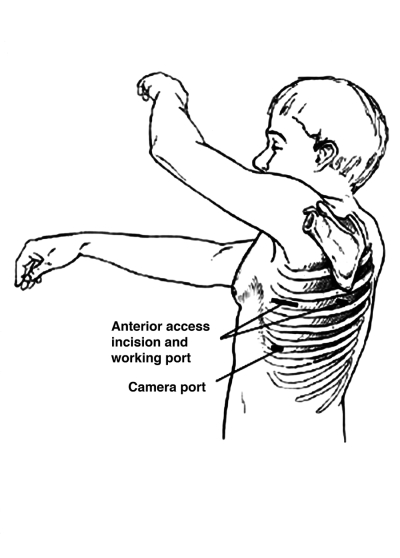
At our institution, VATS lobectomy is conducted with general anesthesia using a double-lumen endotracheal tube. The patient is positioned on the operating table in the lateral decubitus position. A standard thoracoscopy is then performed with three 10-mm incisions: a camera port is placed in the anterior axillary line in the 7th or 8th intercostal space; an anterior port, between the latissimus dorsi and pectoralis major muscles in the 4th or 5th intercostal space; and a posterior port, adjacent to the scapula in the 5th or 6th intercostal space. Through these working thoracoscopy ports, an exploratory thoracoscopy is performed. Subsequently, the anterior thoracoscopy incision is extended to create a 3- to 5-cm muscle-sparing access incision (Figure) to proceed with VATS lobectomy and allow for removal of the lobectomy specimen. Rib spreading and rib retractors are avoided. As in open lobectomy, the pulmonary vein branches from the targeted lobe are identified and dissected before their division using an endoscopic stapling device. Pulmonary artery branches and the bronchus are identified, dissected, stapled, and divided in a similar manner. The specimen is removed from the pleural space within an endoscopic bag to avoid port-site contamination. After the lobectomy is completed, a hilar and mediastinal lymphadenectomy or lymph node sampling is performed. Hemostasis and bronchial stump integrity are confirmed before closure. The incisions are closed after a chest tube is placed via the inferior thoracoscopy port. The chest tube is connected to the water seal via a pleural collection device.
FIGURE.
Video-assisted thorascopic lobectomy incisions.
Depending on the chosen mode of pain management, an epidural catheter is placed before surgery or intravenous patient-controlled analgesia is used after surgery once intraoperative intercostal nerve blocks have been placed.
Statistical Analyses
The SPSS software package 14.0 for Windows (SPSS, Chicago, IL) was used for statistical analyses. Data are presented as median with minimum and maximum values and interquartile range (IQR) for continuous variables and percentages for dichotomous variables.
RESULTS
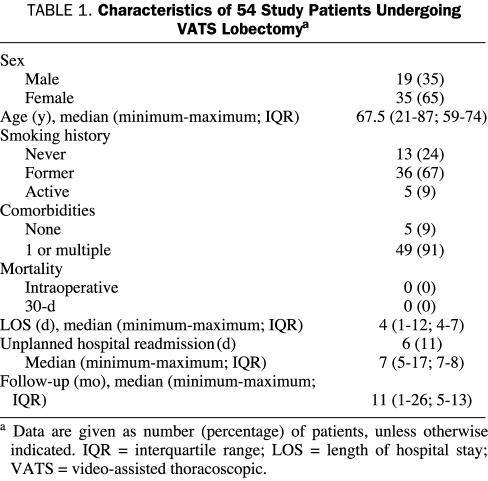
Of the 54 study patients, 19 (35%) were men and 35 (65%) were women. Their median age was 67.5 years (minimum-maximum, 21-87 years; IQR, 59-74 years). Table 1 describes overall patient characteristics.
TABLE 1.
Characteristics of 54 Study Patients Undergoing VATS Lobectomya
A smoking history was obtained before pulmonary resection in all patients in accordance with our institutional thoracic surgery quality standards29; 13 patients (24%) were never smokers, 36 (67%) were former smokers and 5 (9%) patients were current smokers or had quit within a month of their operation. All current smokers were counseled on smoking cessation and offered referral to our institution's smoking cessation program.29 Among the former and active smokers, pack-year history varied from 1.5 up to 150 pack-years.
There were only 5 patients (9%) who had no noted comorbidities. Thirty-seven patients (69%) had cardiovascular comorbidities, such as coronary artery disease, prior myocardial infarction, stroke or transient ischemic attack, or peripheral vascular disease. Seven patients (13%) had substantial chronic pulmonary obstructive disorder. Other frequent comorbidities documented were hypertension (35%), dyslipidemia (32%), and former malignancies (22%).
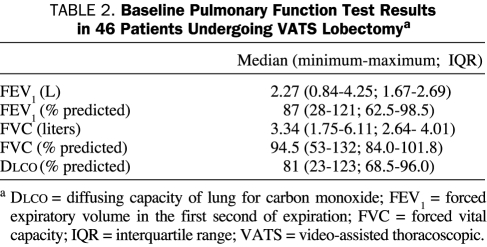
Complete preoperative pulmonary function tests consisting of forced expiratory volume in the first second of expiration, forced vital capacity, and the diffusing capacity of the lung for carbon monoxide were obtained in 46 patients (85%). Detailed baseline pulmonary function test results are summarized in Table 2.
TABLE 2.
Baseline Pulmonary Function Test Results in 46 Patients Undergoing VATS Lobectomya
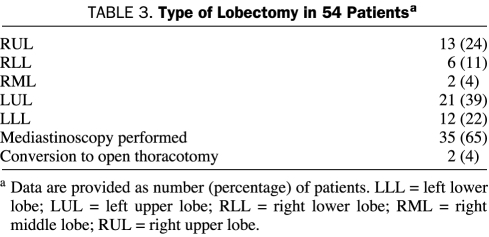
A mediastinoscopy was performed in 35 patients (65%) during the same operation before thoracoscopy and VATS lobectomy. A VATS wedge excisional biopsy was performed for diagnosis before completion of VATS lobectomy in 20 patients (37%). Median duration of surgery for the VATS lobectomy alone was 139 minutes (minimum-maximum, 78-275 minutes; IQR, 121-182 minutes). In cases preceded by a VATS wedge excisional biopsy, the median operative time was 171 minutes (minimum-maximum, 114-304 minutes; IQR, 132-190 minutes). Conversion to an open procedure was necessary in 2 patients because of bleeding from the pulmonary artery.
The types of lobectomies performed and their frequency are shown in Table 3. The median estimated blood loss was 150 mL. One patient, who was converted to an open lobectomy, required intraoperative transfusion with blood products (red blood cells and fresh frozen plasma) because of considerable blood loss.
TABLE 3.
Type of Lobectomy in 54 Patientsa
A single chest tube was inserted in 52 patients (96%). Two chest tubes were used for the 2 patients converted to an open lobectomy.
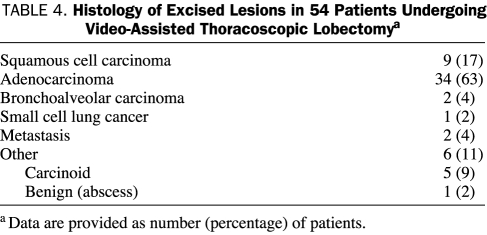
Histology of the excised lesions is shown in Table 4. The median tumor size was 2.2 cm (minimum-maximum, 0.5-5.0 cm; IQR, 1.6-3.0 cm). According to the final pathology reports of patients undergoing surgery for non-small cell lung cancer (NSCLC) (n=45), pathological stage IA was found in 28 patients (62%), IB in 12 (27%), IIA in 4 (9%), and IIB in 1 (2%). Five patients were diagnosed as having stage I carcinoid tumors (4 were identified as typical carcinoids and 1 was unspecified), and 1 patient was diagnosed as having limited small cell lung cancer on final pathology. Two resections were conducted for a solitary metastasis not otherwise amenable to limited resection: one for a colon cancer recurring 5 years after the resection of the primary colon tumor and one for a lesion diagnosed as metastatic renal cell cancer that recurred 12 years after resection of the primary renal cell tumor. One patient was diagnosed as having benign disease on final pathology, showing an organizing pneumonia with an adjacent inflammatory abscess cavity. Data on collected lymph nodes were available for all 54 patients. The median number of total collected lymph nodes was 16.5 (minimum-maximum, 6-51; IQR, 11.3-22.0). In patients who underwent mediastinoscopy, a median of 19 lymph nodes (minimum-maximum, 6.0-51.0; IQR, 15.5-25.0 lymph nodes) were collected, whereas in those patients who did not undergo mediastinoscopy the median number of lymph nodes obtained was 11.5 (minimum-maximum, 6-41; IQR, 8-14). Final pathology confirmed complete resection without microscopic residual tumor (R0-resection) in 100% of patients with malignant disease (n=53). One re-resection of the bronchial margin at the time of the operation was necessary in a patient with a carcinoid tumor in order to obtain R0-resection because pathological examination revealed a second focus of carcinoid tumor in the resected specimen separate from the primary tumor.
TABLE 4.
Histology of Excised Lesions in 54 Patients Undergoing Video-Assisted Thoracoscopic Lobectomya
Pain management consisted of patient-controlled analgesia in 42 patients (78%), an epidural catheter in 3 patients (6%), and both in 9 patients (17%).
There was no operative or 30-day mortality. Of the study patients, 35 (65%) had no postoperative complications. The postoperative course of 4 patients (7%) was complicated by a prolonged air leak (>7 days), 1 of which required another operation. Three of these patients were discharged from the hospital with a Heimlich valve for further management. Postoperative atrial fibrillation occurred in 3 patients (6%) and sinus tachycardia in 1 (2%). All were responsive to antiarrhythmic medical therapy. Other observed complications were urinary tract infection or transitory urinary retention in 3 patients (6%), ileus in 2 patients (4%), and chyle leak, pneumothorax, delirium, and bilateral effusions/pulmonary edema requiring diuresis in 1 patient each (2%).
Chest tubes were removed after a median of 2 days after surgery (minimum-maximum, 1-12 days; IQR, 1.3-3.8 days); as already noted, 3 of the patients from whom chest tubes were removed were converted to a Heimlich valve after 3 to 12 days. The chest tube was removed on postoperative day 1 in 14 patients (26%) and on postoperative day 2 in 15 patients (28%).
Patients were discharged a median of 4 days after their surgery (minimum-maximum, 1-12 days; IQR, 4-7 days). Discharge occurred on postoperative day 2 in 7 patients (13%) and on postoperative day 3 in 12 patients (22%). Unplanned readmission to the hospital within 30 days of postoperative discharge occurred in 6 patients (11%) at a median of 7 days after discharge (minimum-maximum, 5-17 days; IQR, 7-8 days). Readmissions were due to pain, shortness of breath, serous wound secretion, pneumonia, empyema, and persistent air leak with subcutaneous emphysema in 1 patient each.
All patients were evaluated by our multidisciplinary lung cancer team. No adjuvant treatment was used in 41 (91%) of the 45 patients with NSCLC. Adjuvant chemotherapy was given to 6 patients: 4 patients with NSCLC, 1 patient with resected small cell lung cancer, and 1 patient with metastatic renal cell cancer. Progressive disease with local recurrence or distant metastases was noted in 2 (4%) of 45 patients. None of the recurrences were in the location of port sites or access incisions.
Median follow-up was 11 months (minimum-maximum, 1-26 months; IQR, 5-13 months). Nine patients (17%) were lost to follow-up and 4 patients (7%) died during the follow-up period. One patient died of progressive distant metastatic disease 13 months after surgical resection of the primary malignancy, 1 patient of ureteral carcinoma 8 months after VATS lobectomy, and 2 patients of unknown causes 2 months after surgery.
DISCUSSION
The American College of Chest Physicians' evidence-based clinical practice guidelines for the treatment of stage I and II NSCLC consider VATS lobectomy to be an acceptable alternative to open thoracotomy.30 The main concern has been to establish whether equivalent oncologic results can be achieved with VATS lobectomy as with conventional open thoracotomy. With VATS lobectomy vs conventional open thoracotomy, an inadequate lymph node dissection is possible, leading to an understaging of patients. Another point of criticism is that a thorough bimanual lung palpation possible during open thoracotomy to rule out further lung nodules is not feasible via the minimally invasive VATS approach. A recent prospective study by Cerfolio and Bryant31 evaluated 166 patients undergoing open thoracotomy for NSCLC potentially removable by VATS. The authors showed that 22% of patients had nodules that were detected intraoperatively by means of bimanual lung palpation and that were missed by preoperative modern imaging; 8% of these were malignant. Although a control group did not exist in this prospective study, the authors conclude from their data that these nodules would have been missed using minimally invasive approaches. The current overall literature suggests that VATS lobectomies provide oncologic outcomes and long-term results comparable with those achieved with open lobectomy.14,18,32 Because of the short follow-up of the cohort in our study, a definite statement on long-term local or distant recurrence cannot be made.
If adjuvant therapy is required, improved tolerance of chemotherapy is another potential benefit of VATS lobectomy compared with lobectomy through a conventional thoracotomy.12,27,33 However, no randomized prospective data are available to support this contention.
Given the rising percentage of elderly people in our society and their ever-increasing life expectancy,34 technological advances are needed to enhance the safety of surgery for elderly patients.35,36 According to the Surveillance, Epidemiology and End Results database of the National Cancer Institute, the median age at diagnosis of lung cancer between 2001 and 2005 was 71 years; 28.9% of the patients were aged 75 through 84 years, whereas 7.3% were aged 85 years and older.37 In our series, 28% of patients were aged 70 to 79 years, and 15% were older than 80 years with no evidence of increased major complications. Cattaneo et al26 showed in their retrospective, matched case-control series that VATS lobectomies in septuagenarians are associated with fewer complications and shorter hospital stays than open thoracotomies. Another retrospective study conducted by Mun and Kohno38 showed that VATS lobectomy used for early stage lung cancer is also a safe and effective procedure in octogenarians with proper patient selection. Likewise, in patients with underlying pulmonary disease or diminished pulmonary function, VATS lobectomies can be performed safely.33,39 In our series, 43% of patients had a preoperative forced expiratory volume in the first second of expiration of 75% or less of that predicted.
We continue to explore the possibility that the candidate pool for surgical lobectomy can be expanded with the advent of more minimally invasive approaches such as VATS lobectomy. Indeed, such an expansion is one of the most exciting possibilities raised by the use of these surgical techniques.
Atrial fibrillation is a common postoperative complication in thoracic surgery with rates of up to 20% reported for open lobectomy.40,41 After VATS lobectomy, rates between 2.9%11 and 10.0%42 have been reported by the 2 largest single-institution case series. McKenna et al27 reported a very low incidence of atrial fibrillation (5 cases, 1.8%) in a series of 282 consecutive patients with a median length of hospital stay of 3 days. This phenomenon, however, might be attributable to the short hospital stays of patients. In our series, consistent with reported data, we observed a postoperative rate of atrial fibrillation of 6%. A lower frequency of atrial fibrillation after minimally invasive lobectomy remains controversial because data from 122 patients studied by Park et al43 suggest an equal frequency of atrial fibrillation compared with lobectomy via open thoracotomy. Park et al concluded from their data that prophylactic antiarrhythmic regimens should be the same for both approaches.
CONCLUSION
Although multi-institutional, prospective, randomized trials have yet to be completed and the size of our retrospective study is limited, the early experience at our institution shows that VATS lobectomy is associated with low perioperative morbidity and mortality and therefore is a feasible and safe approach to the surgical treatment of lung cancer. The associated short hospital stays of patients may have an effect on quality of life and overall health care costs for the treatment of this disease.
REFERENCES
- 1.Allen MS, Deschamps C, Jones DM, Trastek VF, Pairolero PC. Video-assisted thoracic surgical procedures: the Mayo experience. Mayo Clin Proc. 1996;71(4):351-359 [DOI] [PubMed] [Google Scholar]
- 2.Allen MS, Deschamps C, Lee RE, Trastek VF, Daly RC, Pairolero PC. Video-assisted thoracoscopic stapled wedge excision for indeterminate pulmonary nodules. J Thorac Cardiovasc Surg. 1993;106(6):1048-1052 [PubMed] [Google Scholar]
- 3.Hazelrigg SR, Nunchuck SK, LoCicero J., III Video assisted Thoracic Surgery Study Group data. Ann Thorac Surg. 1993;56(5):1039-1043 [DOI] [PubMed] [Google Scholar]
- 4.Lewis RJ, Caccavale RJ, Sisler GE, Mackenzie JW. One hundred consecutive patients undergoing video-assisted thoracic operations. Ann Thorac Surg. 1992;54(3):421-426 [DOI] [PubMed] [Google Scholar]
- 5.Miller DL, Allen MS. Set-up and present indications: video-assisted thoracic surgery. Semin Thorac Cardiovasc Surg. 1993;5(4):280-283 [PubMed] [Google Scholar]
- 6.Coltharp WH, Arnold JH, Alford WC, Jr, et al. Videothoracoscopy: improved technique and expanded indications. Ann Thorac Surg. 1992;53(5):776-778 [DOI] [PubMed] [Google Scholar]
- 7.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008February;135(2):247-254 Epub 2007 Dec 21 [DOI] [PubMed] [Google Scholar]
- 8.Congregado M, Merchan RJ, Gallardo G, Ayarra J, Loscertales J. Video-assisted thoracic surgery (VATS) lobectomy: 13 years' experience. Surg Endosc. 2008August;22(8):1852-1857 Epub 2007 Dec 20 [DOI] [PubMed] [Google Scholar]
- 9.Kirby TJ, Rice TW. Thoracoscopic lobectomy. Ann Thorac Surg. 1993;56(3):784-786 [DOI] [PubMed] [Google Scholar]
- 10.Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy—video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial. J Thorac Cardiovasc Surg. 1995;109(5):997-1001 [DOI] [PubMed] [Google Scholar]
- 11.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81(2):421-425 [DOI] [PubMed] [Google Scholar]
- 12.Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135(3):642-647 [DOI] [PubMed] [Google Scholar]
- 13.Sakuraba M, Miyamoto H, Oh S, et al. Video-assisted thoracoscopic lobectomy vs. conventional lobectomy via open thoracotomy in patients with clinical stage IA non-small cell lung carcinoma. Interact Cardiovasc Thorac Surg. 2007October;6(5):614-617 Epub 2007 Jul 26 [DOI] [PubMed] [Google Scholar]
- 14.Solaini L, Prusciano F, Bagioni P, Poddie DB. Long-term results of video-assisted thoracic surgery lobectomy for stage I non-small cell lung cancer: a single-centre study of 104 cases. Interact Cardiovasc Thorac Surg. 2004;3(1):57-62 [DOI] [PubMed] [Google Scholar]
- 15.Solaini L, Prusciano F, Bagioni P, di Francesco F, Solaini L, Poddie DB. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc. 2008February;22(2):298-310 Epub 2007 Oct 18 [DOI] [PubMed] [Google Scholar]
- 16.Swanson SJ, Herndon JE, II, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802-a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25(31):4993-4997 [DOI] [PubMed] [Google Scholar]
- 17.Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83(6):1965-1970 [DOI] [PubMed] [Google Scholar]
- 18.Lewis RJ, Caccavale RJ, Sisler GE, Mackenzie JW. Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiovasc Surg. 1992;104(6):1679-1685 [PubMed] [Google Scholar]
- 19.McKenna RJ., Jr Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg. 1994;107(3):879-881 [PubMed] [Google Scholar]
- 20.Giudicelli R, Thomas P, Lonjon T, et al. Major pulmonary resection by video assisted mini-thoracotomy: initial experience in 35 patients. Eur J Cardiothorac Surg. 1994;8(5):254-258 [DOI] [PubMed] [Google Scholar]
- 21.Alam N, Flores RM. Video-assisted thoracic surgery (VATS) lobectomy: the evidence base. JSLS 2007;11(3):368-374 [PMC free article] [PubMed] [Google Scholar]
- 22.Sawada S, Komori E, Yamashita M, et al. Comparison in prognosis after VATS lobectomy and open lobectomy for stage I lung cancer: retrospective analysis focused on a histological subgroup. Surg Endosc. 2007September;21(9):1607-1611 Epub 2007 Feb 16 [DOI] [PubMed] [Google Scholar]
- 23.Rocco G, Internullo E, Cassivi SD, Van Raemdonck D, Ferguson MK. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin. 2008;18(3):235-247 [DOI] [PubMed] [Google Scholar]
- 24.Allen MS, Darling GE, Pechet TT, et al. ACOSOG Z0030 Study Group Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81(3):1013-1019 [DOI] [PubMed] [Google Scholar]
- 25.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56(6):1285-1289 [DOI] [PubMed] [Google Scholar]
- 26.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85(1):231-235 [DOI] [PubMed] [Google Scholar]
- 27.McKenna RJ, Jr, Mahtabifard A, Pickens A, Kusuanco D, Fuller CB. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. Ann Thorac Surg. 2007;84(5):1663-1667 [DOI] [PubMed] [Google Scholar]
- 28.Petersen RP, Pham D, Burfiend WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83(4):1245-1249 [DOI] [PubMed] [Google Scholar]
- 29.Cassivi SD, Allen MS, Vanderwaerth GD, et al. Patient-centered quality indicators for pulmonary resection. Ann Thorac Surg. 2008;86:927-932 [DOI] [PubMed] [Google Scholar]
- 30.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(3 suppl):234S-242S [DOI] [PubMed] [Google Scholar]
- 31.Cerfolio RJ, Bryant AS. Is palpation of the nonresected pulmonary lobe(s) required for patients with non-small cell lung cancer? A prospective study. J Thorac Cardiovasc Surg. 2008;135(2):261-268 [DOI] [PubMed] [Google Scholar]
- 32.Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg. 2008;85(2):S710-S715 [DOI] [PubMed] [Google Scholar]
- 33.Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg. 2008;85(2):S719-S728 [DOI] [PubMed] [Google Scholar]
- 34.Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature 2008February7;451(7179):716-719 [DOI] [PubMed] [Google Scholar]
- 35.Dominguez-Ventura A, Allen MS, Cassivi SD, Nichols FC, III, Deschamps C, Pairolero PC. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg. 2006;82(4):1175-1179 [DOI] [PubMed] [Google Scholar]
- 36.Dominguez-Ventura A, Cassivi SD, Allen MS, et al. Lung cancer in octogenarians: factors affecting long-term survival following resection. Eur J Cardiothorac Surg. 2007August;32(2):370-374 Epub 2007 Jun 6 [DOI] [PubMed] [Google Scholar]
- 37.National Cancer Institute http://seer.cancer.gov. Surveillance Epidemiology and End Results (SEER) web site. Accessed March 2, 2009.
- 38.Mun M, Kohno T. Video-assisted thoracic surgery for clinical stage I lung cancer in octogenarians. Ann Thorac Surg. 2008;85(2):406-411 [DOI] [PubMed] [Google Scholar]
- 39.Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg. 2008;85(2):S705-S709 [DOI] [PubMed] [Google Scholar]
- 40.Rena O, Papalia E, Oliaro A, et al. Supraventricular arrhythmias after resection surgery of the lung. Eur J Cardiothorac Surg. 2001;20(4):688-693 [DOI] [PubMed] [Google Scholar]
- 41.Lanza LA, Visbal AI, DeValeria PA, Zinsmeister AR, Diehl NN, Trastek VF. Low-dose oral amiodarone prophylaxis reduces atrial fibrillation after pulmonary resection. Ann Thorac Surg. 2003;75(1):223-230 [DOI] [PubMed] [Google Scholar]
- 42.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244(3):420-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park BJ, Zhang H, Rusch VW, Amar D. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg. 2007;133(3):775-779 [DOI] [PubMed] [Google Scholar]