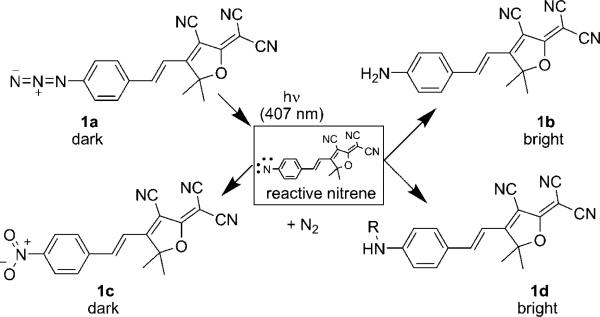
Scheme 1.
Photoactivation reactions of the azido DCDHF fluorogen. Aryl azides are known to be photolabile; the loss of dinitrogen leaves a reactive nitrene intermediate, which can rearrange to form a seven-membered azepine heterocycle (not shown), an amine (1b), or a nitro (1c) group. Compounds 1a and 1c are not fluorescent when pumped at long wavelengths, but photoproducts 1b and 1d are fluorescent. Compound 1d is hypothetical and the result of nitrene inserting into C–C bonds of a nearby biomolecule. (Adapted with permission from J. Am. Chem. Soc. 2008, 130, 9204−9205. Copyright 2008 American Chemical Society.)