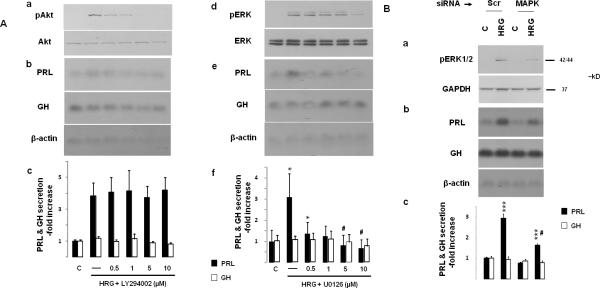
Fig. 5. ERK signaling mediates heregulin-induced PRL.
A) GH4C1 cells were serum-starved overnight and pre-treated for 45 min with the PI3K inhibitor LY294002 (Aa-c) or the MEK inhibitor U0126 (Ad-f). Cells were treated with HRG (6 nM) for 10 min (Aa and Ad) or 48 hrs (Ab-c, Ae-f). Phosphorylated Akt and total Akt (Aa), as well as phosphorylated ERK and total ERK (Ad) were detected by Western blot in whole protein extracts. GH and PRL mRNA expression and protein secretion were determined by Northern blot (Ab, Ae) and RIA (Ac, Af) as for Fig. 1. A representative of 3 independently performed experiments is shown (mean ± SE). *, p<0.05 vs. control group; #, p<0.05 vs. HRG only (-) group.
B) GH4C1 cells were serum-starved overnight, transfected with negative control (Scr) or rat MAPK1 (100 pmol) siRNA (24 hrs). After an additional 8 hr (serum-free medium), cells were treated with HRG (6 nM) for 10 min (Ba) or 48 hrs (Bb-c). Phosphorylated ERK and GAPDH loading control were detected by Western blot in whole protein extracts. GH and PRL mRNA expression and protein secretion were determined by Northern blot and RIA as for Fig. 1. A representative of 3 independently performed experiments is shown (mean ± SE). ***, p<0.001 vs. Scr siRNA control group; #, p<0.001 vs. Scr HRG group.