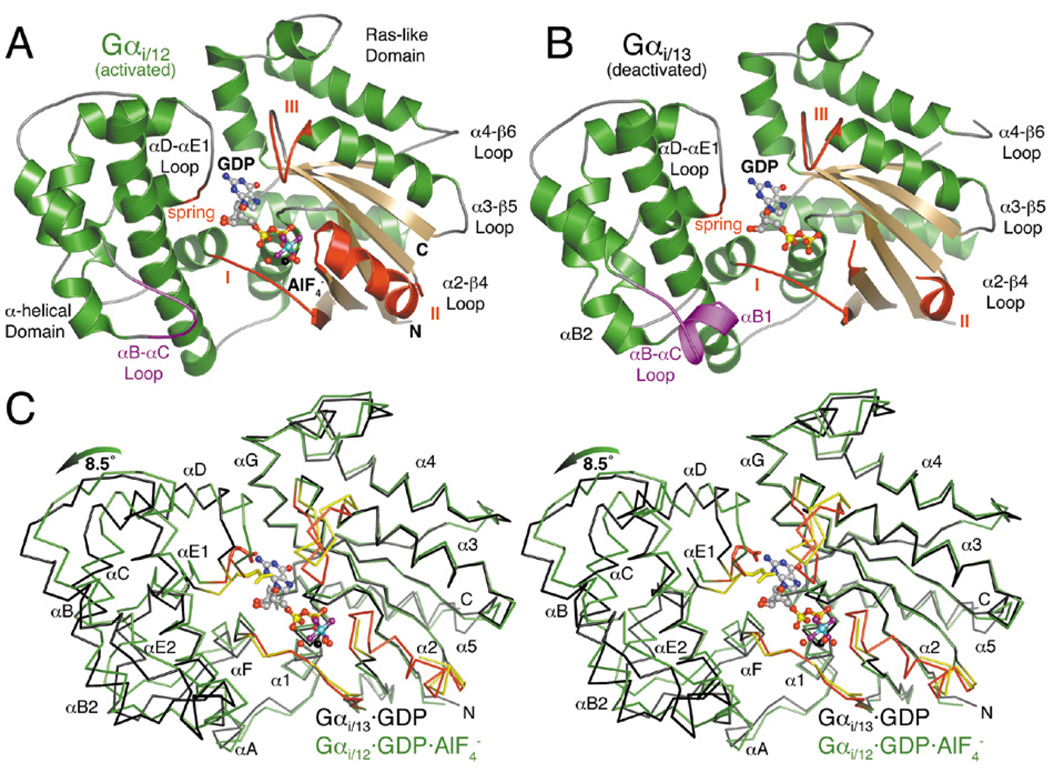
Figure 4.
Structures of Gα12/13 subunits in activated and deactivated states. (A) The activated Gαi/12·GDP·AlF4− complex. The region of the αB-αC loop distinct from that of Gαi/13 is colored purple. The three conformationally flexible “switch regions” of the Gα subunit are red and labeled with Roman numerals (I–III). A fourth, apparently Gα12/13-specific element (the “spring”) is likewise colored red. The Mg2+·GDP·AlF4− ligand complex is shown as a ball-and-stick model, with carbons colored gray, oxygens red, nitrogens blue, phosphates yellow, fluorines purple, aluminum cyan and magnesium black. Waters are shown as red spheres. (B) The deactivated Gαi/13·GDP complex adopts an unusually open conformation in which the α-helical domain has rotated ~8.5° away from the Ras-like domain. Switch II is almost completely disordered, and switch III has rotated away (up in the figure) from the nucleotide binding site. (C) Stereo view of the Cα traces of Gαi/12·GDP·AlF4− (green with red switch regions) and Gαi/13·GDP (black with yellow switch regions), superimposed using their Ras-like domains. The glutamate side chain in the “spring” of each α-helical domain (E175 in Gα12, E172 in Gα13) is shown as a stick model.