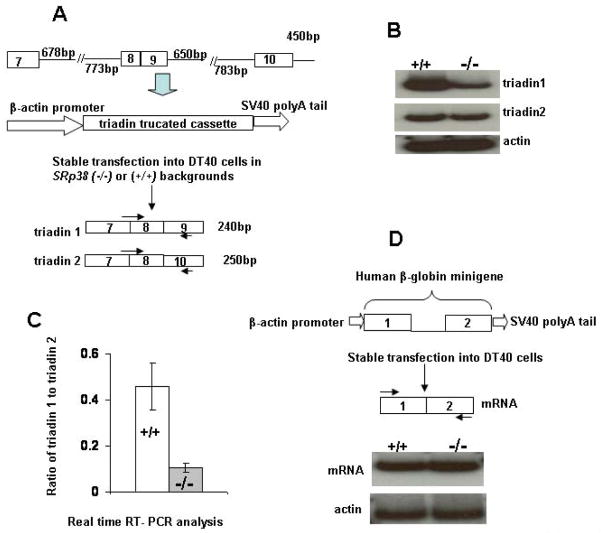
Figure 6. SRp38 enhances inclusion of triadin exon 9 in stably transfected DT40 cells.
(A) Diagram of reporter plasmid containing the indicated triadin genomic sequences, procedure for stable transfection of the reporter plasmid into SRp38(+/+) and SRp38(−/−) DT40 cell lines, and the two alternatively spliced triadin products. The forward primer was designed to span the junction of exons 7–8 in order to detect only spliced products. (B) SRp38 depletion decreases the level of triadin 1 mRNA in SRp38(−/−) DT 40 cells. RNA was extracted from SRp38(+/+) and SRp38(−/−) cells and 32P RT-PCR was performed. RNA was resolved on a 6% denaturing PAGE and triadin 1 and triadin 2 products were shown. Actin mRNA levels were used as a loading control. (C) The ratio of triadin 1 to triadin 2 mRNA levels is greatly reduced in SRp38(−/−) DT40 cells. RNA was extracted from SRp38(+/+) and SRp38(−/−) DT40 cell clones stably expressing the triadin reporter construct. Real-time RT-PCR analysis was performed as described in Figure 4A. Comparison was made between five independent SRp38(+/+) and five independent SRp38(−/−) DT40 cell clones. (D) Basal splicing activity in SRp38(+/+) and SRp38(−/−) DT40 cells. A plasmid containing the human β-globin gene was constructed and stably transfected into DT 40 cells following the same strategy employed in (A). RNA was extracted and 32P RT-PCR was performed.