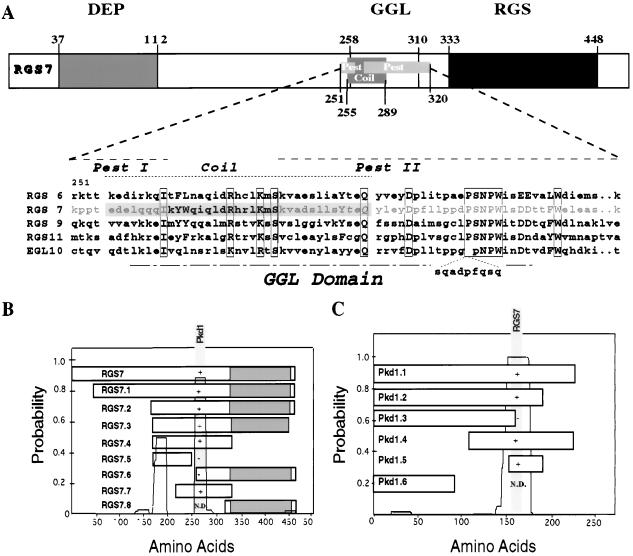
Figure 1.
Sequence analysis of human RGS7 and interaction with polycystin in yeast. (A) RGS7 shares a common domain structure with the mammalian RGS6, RGS9, RGS11, and EGL-10. Depicted in detail is the region unique to RGS7, containing two PEST sequences and a potential coiled-coil structure. The similarity and identity of the RGS7 GGL domain versus that of RGS6, 9, 11, and EGL-10 were 81% and 55%, 60% and 37%, 58% and 33%, and 59% and 40%, respectively. (B) Mutational analysis of RGS7–polycystin interactions in yeast. The presence of coiled-coil structures is depicted as a probability plot by using coils Version 2.1 from the Swiss Institute for Experimental Cancer Research, window 28. Interactions between RGS7 and polycystin were tested in the yeast two-hybrid system, with interaction (+) indicated by β-galactosidase production and leucine prototrophy. Interaction between RGS7 and polycystin is mediated by a region of RGS7 that contains a putative coiled-coil structure (shaded region). (C) Deletion of the C-terminal 70 aa of polycystin (Pkd1.3) abolishes binding to RGS7. Pkd1.1 represents the C-terminal 226 aa of polycystin. The minimal interacting domain, Pkd1.5, caused leucine prototrophy without β-galactosidase production, indicative of a weak interaction. The putative binding domain within polycystin contains a coiled-coil structure, L4215–R4249. N.D., interaction not tested.