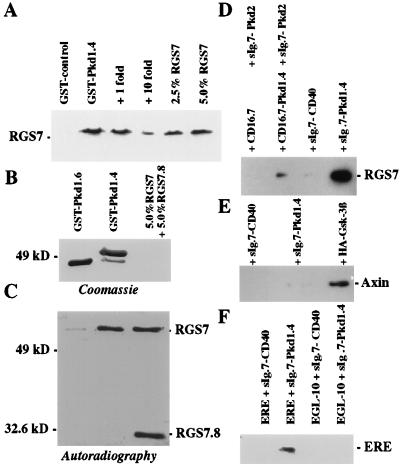
Figure 2.
RGS7 associates with polycystin in vitro and in vivo. (A) In vitro binding of RGS7 to C-terminal polycystin. The amount of [35S]methionine-labeled RGS7 that bound to the last 112 aa of polycystin fused to GST (GST–Pkd1.4) or to GST–FIT, an unrelated control peptide of equal length, was compared with 2.5% and 5% 35S-labeled RGS7 input protein. The interaction between polycystin and [35S]methionine-labeled RGS7 was blocked by increasing concentrations of unlabeled RGS7. (B and C) In vitro interaction of RGS7 and RGS7.8 with GST–Pkd1.4 and GST–Pkd1.6, respectively. The GST fusion proteins were immobilized to glutathione–Sepharose and incubated with a mixture of equal amounts of RGS7 and RGS7.8 (5% input proteins). RGS7 binds to GST–Pkd1.4, but not to GST–Pkd1.6; no binding to either GST fusion was detectable for RGS7.8 (lacking the PKD1-interacting domain) compared with 5% input proteins. (D) In vivo coimmunoprecipitation of F.RGS7 with C-terminal polycystin. The fragment of polycystin containing the last 112 aa (Pkd1.4) was fused to sIg.7, a construct containing the leader sequence of human CD5 followed by the CH2 and CH3 domain of human IgG1 and the transmembrane region of human CD7. The control plasmid, sIg.7-CD40 contains the cytoplasmic domain of CD40 following the end of the transmembrane region of CD7. For interaction between polycystin and RGS7, 293T cells were transiently transfected with F.RGS7 and either sIg.7-CD40 (control) or sIg.7-Pkd1.4. Lysates were immunoprecipitated with protein G and blotted with anti-Flag M2 mAb. To demonstrate that RGS7 binds the polycystin-PKD2 complex, 293T cells were transiently transfected with F.RGS7 and sIg.7-PKD2, an IgG-fusion protein containing the C-terminal cytoplasmic domain of PKD2 (27), and either CD16.7 or CD16.7-Pkd1.4 (27). Lysates were immunoprecipitated with protein G and blotted with anti-Flag M2 mAb. (E) The coiled-coil RGS protein axin binds GSK-3β, but does not interact with polycystin. sIg.7-CD40, sIg.7-Pkd1.4, or HA-tagged GSK-3β were transfected with myc-tagged axin and immunoprecipitated with protein G or a combination of anti-HA mAb and protein G. HA–GSK-3β, but neither sIg.7-CD40 nor sIg.7-Pkd1.4, precipitated axin. (F) EGL-10, a C. elegans homologue lacking a coiled-coil structure, does not interact with the C terminus of polycystin. However, a Flag-tagged chimera of EGL-10 containing the polycystin-binding domain of RGS7 (F.ERE), coprecipitated with sIg.7-Pkd1.4 but not with sIg.7-CD40.