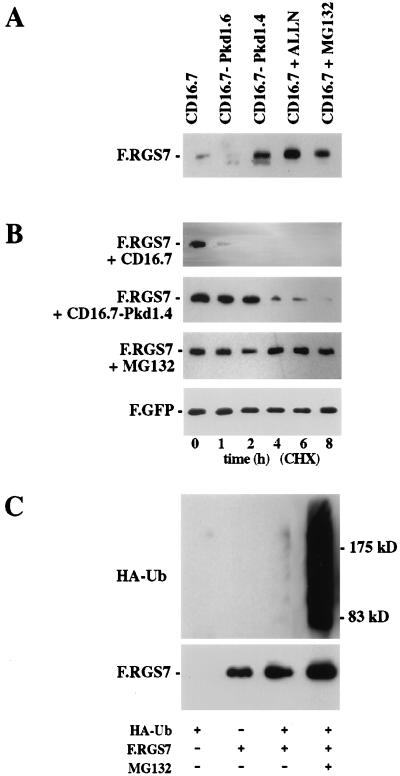
Figure 3.
Effect of polycystin on RGS7 protein levels. (A) RGS7 protein expression is augmented by the presence of polycystin or proteasome inhibitors. 293T cells were transfected with F.RGS7 and cotransfected with CD16.7-Pkd1.4 or controls (CD16.7 and CD16.7-Pkd1.6). After 24 hours, cells were incubated for 3 hours in medium alone (lanes 1–3), or with N-Acetyl-Leu-Leu-Norleucinal (ALLN) (40 μmol) or MG132 (40 μmol). Equal amounts of protein were separated by using SDS/PAGE, and Flag-tagged proteins were detected by Western blot analysis. (B) RGS7 protein half-life is increased in the presence of polycystin or a proteasome inhibitor. 293T cells were cotransfected with F.RGS7 and CD16.7.Pkd1.4 or CD16.7 (control). After 24 hours, cells were incubated 3 hours in medium alone or with MG132 (40 μmol). Cells were harvested at the time points indicated after the addition of cycloheximide (40 μg/ml), and whole cell lysates were immunoblotted with anti-Flag M2 to detect F.RGS7. Equal transfection efficiency and amount of loaded protein was monitored by a Flag-tagged green fluorescent protein construct (data shown for F.RGS7 + CD16.7). (C) Ubiquitination of RGS7 is increased in the presence of a proteasome inhibitor. HEK 293T cells were transfected with F.RGS7 and HA–ubiquitin as indicated. Flag/His-tagged RGS7 was purified by nickel-NTA chromatography from lysates of transfected cells, run on a SDS/PAGE gel, and transferred to nitrocellulose membrane. RGS7-ubiquitin conjugates were detected with anti-HA antibodies and F.RGS7 detected with the anti-Flag M2 antibody.