Figure 4.
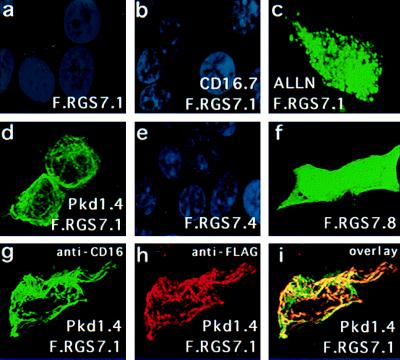
Colocalization of RGS7 with polycystin in 293T cells. Cells were transiently transfected with expression plasmids encoding F.RGS7.1 (a–d and g–i), F.RGS7.4 (e), F.RGS7.8 (f), CD16.7 (b), and CD16.7-Pkd1.4 (d and g–i). Shown are confocal images (Bio-Rad) after labeling of RGS7 with anti-Flag M2 and FITC-conjugated goat anti-mouse IgG (a–f). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI). Cells were transfected with RGS7.1 alone (a) or with the control plasmid CD16.7 (b) and were virtually negative other than the DAPI-labeled nuclei. Addition of ALLN at 40 μmol for 6 h resulted in significant augmentation of RGS7 protein levels and a punctate staining pattern (c). Cells cotransfected with F.RGS7.1 and CD16.7-Pkd1.4 showed a marked augmentation of F.RGS7.1 protein levels and a membrane-associated cage-like staining pattern (d). Deletion of the RGS domain (F.RGS7.4) did not elevate expression levels of RGS7 (e). Deletion of the N-terminal domain, including the PKD1-binding domain, resulted in increased and diffuse cytoplasmic expression of the RGS7 truncation (F.RGS7.8) (f). (g–i) Colocalization of RGS7 and polycystin in 293T cells transfected with F.RGS7.1 and CD16.7-Pkd1.4. CD16.7-Pkd1.4 was labeled with FITC-conjugated anti-CD16 mAb (g). F.RGS7 was labeled with anti-Flag M2 followed by rhodamine-conjugated anti-mouse IgG (h). An overlay shows the colocalization of RGS7 and polycystin expression (i). Identical results were obtained with full-length RGS7, indicating that the N-terminal domain does not affect the polycystin-mediated relocalization of RGS7 (data not shown).