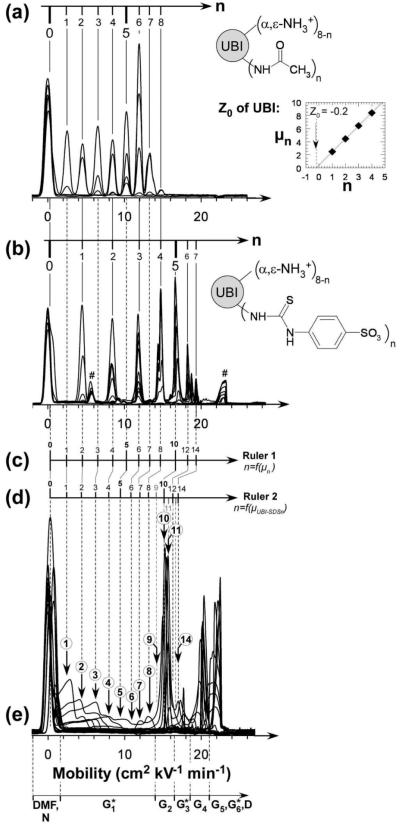
Figure 4.
Superposed electropherograms of charge ladders of ubiquitin obtained respectively by acylation of ε-lysine residues with (a) acetic anhydride and (b) 4-sulfophenylisothiocyanate. Peaks marked with (#) disappear after extensive dialysis. The buffer is tris-glycine, pH 8.4. The inset in panel a corresponds to the plot of the electrophoretic mobilities of the rungs of the acetyl charge ladder of UBI as a function of acetylations n and allows the determination of the net charge of native UBI as a function of charge increment upon acylation, ΔZ. (c) Ruler 1 correlates experimental mobilities μn of the rungs of a UBI charge ladder to the number of acylations n. (d) Ruler 2 correlates the corrected mobilities (μUBI-SDSn, eq 4) of rungs of charge ladders to the number of acylations n. This second ruler permits the determination of the stoichiometry of UBI-SDSn complexes in panel e. Steps in gray in Ruler 2 were extrapolated from steps in black. (e) Superposition of CE electropherograms shown in Figure 1. Labels in circles indicate the stoichiometries of UBI-SDSn complexes within G1*, G2, and G3* according to Ruler 2.