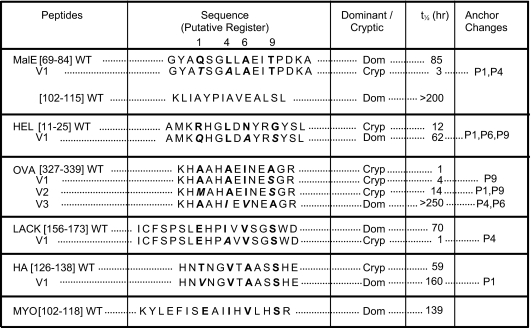
Table 1.
Kinetic stability of wild-type and variant peptide epitopes in association with I-Ad
Half-lifes of peptide:I-Ad complexes were calculated from the time required to dissociate 50% of the FITC-labeled peptide initially bound to soluble I-Ad at pH 7.4. The immunodominance of different peptide epitopes was determined from protein immunizations and denoted as Dom or Cryp, respectively. Putative pocket residues for peptides are indicated in bold, and the mutations at pocket residues that modulate kinetic stability are indicated in bold italics. Lys in OVA[327-339] was substituted for Val to eliminate binding of an alternate register.