Abstract
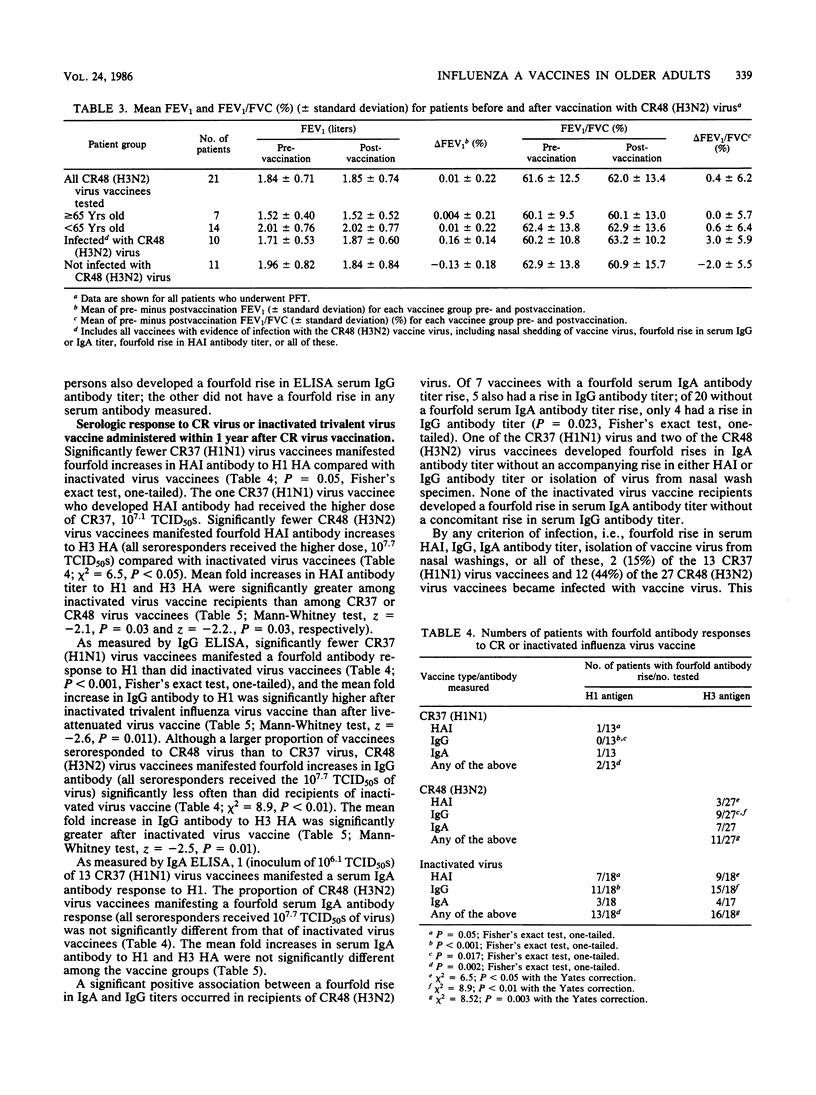
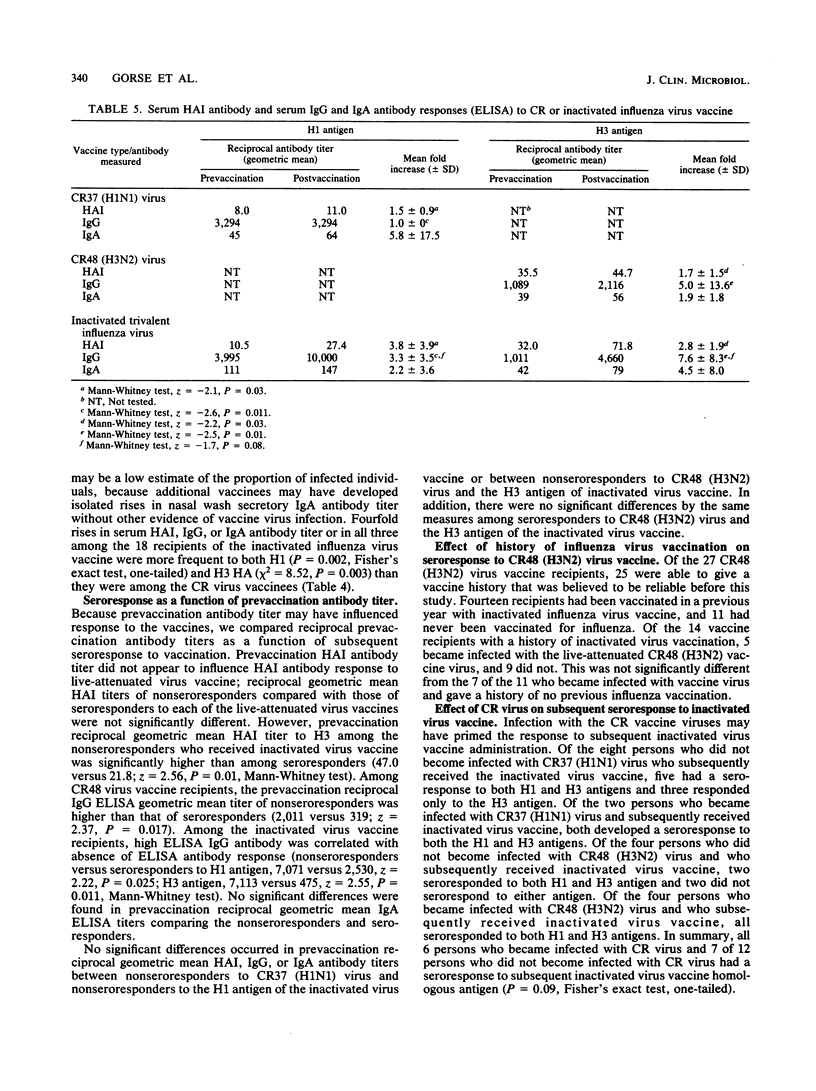
Forty older adults with chronic diseases were vaccinated intranasally with either influenza A/California/10/78 (H1N1) (CR37) or influenza A/Washington/897/80 (H3N2) (CR48) virus. No clinically significant morbidity or decrement in pulmonary function occurred postvaccination. Two (15%) recipients of CR37 virus and twelve (44%) recipients of CR48 virus became infected with vaccine virus, as indicated by a fourfold rise in serum hemagglutination inhibition antibody titer; a fourfold rise in serum immunoglobulin G (IgG) or IgA antibody titer, indicated by enzyme-linked immunosorbent assay; isolation of vaccine virus from nasal washings; or all of these. Within 1 year after cold-recombinant vaccine virus vaccination, 18 vaccines received inactivated trivalent influenza virus vaccine parenterally. Of the vaccinees, 13 (72%) developed a fourfold rise in serum antibody titer to H1N1 antigen and 16 (89%) developed a fourfold rise in serum antibody titer to H3N2 antigen. We conclude that administration of these cold-recombinant vaccine viruses to older adults with chronic diseases was safe, but that serum antibody response rates were lower than those achieved with subsequently administered inactivated influenza virus vaccine given parenterally. However, the higher seroconversion rates attained by using the inactivated trivalent influenza virus vaccine do not necessarily mean that it is more efficacious in preventing infection or severe illness or both due to natural wild-type influenza A virus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker W. H., Mullooly J. P. Influenza vaccination of elderly persons. Reduction in pneumonia and influenza hospitalizations and deaths. JAMA. 1980 Dec 5;244(22):2547–2549. [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Bartram J., Crookshanks F. K. Live attenuated influenza A virus vaccines in children: results of a field trial. J Infect Dis. 1984 Dec;150(6):834–840. doi: 10.1093/infdis/150.6.834. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P. Cold-recombinant influenza A/California/10/78 (H1N1) virus vaccine (CR-37) in seronegative children: infectivity and efficacy against investigational challenge. J Infect Dis. 1984 May;149(5):735–740. doi: 10.1093/infdis/149.5.735. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Brandriss M. W., Betts R. F., Mathur U., Douglas R. G., Jr Responses of elderly subjects to monovalent A/USSR/77 (H1N1) and Trivalent A/USSR/77 (H1N1)-A/TEXAS/77 (H3N2)-B/Hong Kong/72 vaccines. Am Rev Respir Dis. 1981 Dec;124(6):681–684. doi: 10.1164/arrd.1981.124.6.681. [DOI] [PubMed] [Google Scholar]
- Brandriss M. W., Schlesinger J. J., Douglas R. G., Jr Responses of elderly subjects to a new subunit influenza virus vaccine. J Infect Dis. 1982 Feb;145(2):277–277. doi: 10.1093/infdis/145.2.277. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Murphy B. R., Radl J., Haaijman J. J., Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985 Aug;22(2):259–264. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Maassab H. F., Murphy B. R. Dose response of influenza A/Washington/897/80 (H3N2) cold-adapted reassortant virus in adult volunteers. J Infect Dis. 1984 May;149(5):814–815. doi: 10.1093/infdis/149.5.814. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Murphy B. R. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet. 1984 Mar 31;1(8379):705–708. doi: 10.1016/s0140-6736(84)92222-0. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Maassab H. F., Kendal A. P. Comparative studies of wild-type and cold-mutant (temperature-sensitive) influenza viruses: nonrandom reassortment of genes during preparation of live virus vaccine candidates by recombination at 25 degrees between recent H3N2 and H1N1 epidemic strains and cold-adapted A/An Arbor/6/60. Virology. 1979 Aug;97(1):190–194. doi: 10.1016/0042-6822(79)90386-6. [DOI] [PubMed] [Google Scholar]
- D'Alessio D. J., Cox P. M., Jr, Dick E. C. Failure of inactivated influenza vaccine to protect an aged population. JAMA. 1969 Oct 20;210(3):485–489. [PubMed] [Google Scholar]
- Foy H. M., Allan I., Blumhagen J. M., Cooney M. K., Hall C., Fox J. P. A/USSR and B/Hong Kong vaccine. Field experiences during an A/Brazil and an influenza B epidemic. JAMA. 1981 May 1;245(17):1736–1740. [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Edmondson W. P., Jr, Rothenberg R., White P. W. A comparison of subcutaneous, nasal, and combined influenza vaccination. I. Antigenicity. Am J Epidemiol. 1971 Jun;93(6):472–479. doi: 10.1093/oxfordjournals.aje.a121281. [DOI] [PubMed] [Google Scholar]
- Kava T., Laitinen L. A. Effects of killed and live attenuated influenza vaccine on symptoms and specific airway conductance in asthmatics and healthy subjects. Allergy. 1985 Jan;40(1):42–47. doi: 10.1111/j.1398-9995.1985.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Little J. W., Douglas R. G., Jr, Hall W. J., Roth F. K. Attenuated influenza produced by experimental intranasal inoculation. J Med Virol. 1979;3(3):177–188. doi: 10.1002/jmv.1890030303. [DOI] [PubMed] [Google Scholar]
- MacKenzie J. S. Influenza subunit vaccine: antibody responses to one and two doses of vaccine and length of response, with particular reference to the elderly. Br Med J. 1977 Jan 22;1(6055):200–202. doi: 10.1136/bmj.1.6055.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Clements M. L., Madore H. P., Steinberg J., O'Donnell S., Betts R., Demico D., Reichman R. C., Dolin R., Maassab H. F. Dose response of cold-adapted, reassortant influenza A/California/10/78 virus (H1N1) in adult volunteers. J Infect Dis. 1984 May;149(5):816–816. doi: 10.1093/infdis/149.5.816. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P. A., Weber J. A., Parker R. A., Hall W. N., Kendal A. P., Bregman D. J., Schonberger L. B. Efficacy of influenza vaccine in nursing homes. Reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985 Feb 22;253(8):1136–1139. [PubMed] [Google Scholar]
- Phelan M. A., Mayner R. E., Bucher D. J., Ennis F. A. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J Biol Stand. 1980;8(3):233–242. doi: 10.1016/s0092-1157(80)80039-4. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Potter C. W., Stuart-Harris C. H. Antibody response to inactivated influenza vaccine given by different routes in patients with chronic bronchopulmonary disease. Thorax. 1973 Nov;28(6):721–728. doi: 10.1136/thx.28.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Kanner R. E., Golden C. A., Klauber M. R., Renzetti A. D., Jr Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J Infect Dis. 1980 Mar;141(3):271–280. doi: 10.1093/infdis/141.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sérié C., Barme M., Hannoun C., Thibon M., Beck H., Aquino J. P. Effects of vaccination on an influenza epidemic in a geriatric hospital. Dev Biol Stand. 1977 Jun 1;39:317–321. [PubMed] [Google Scholar]


