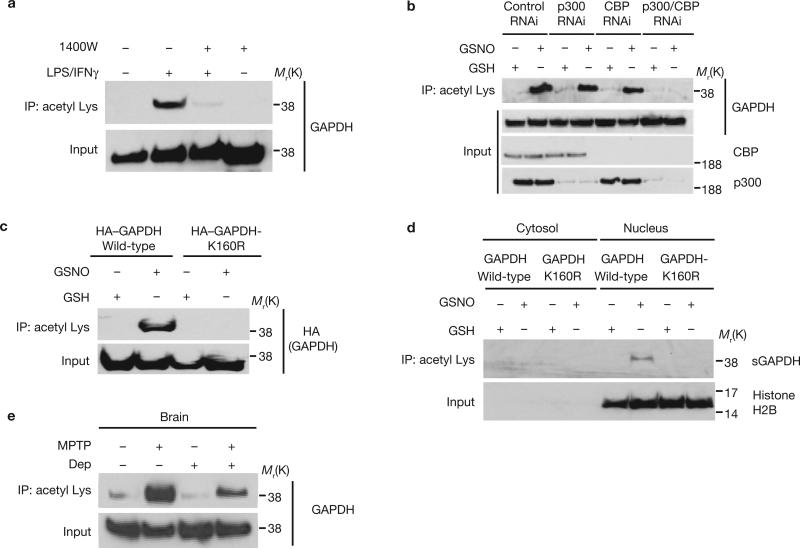
Figure 1.
GAPDH is acetylated in the nucleus at Lys 160 following NO stimulation. (a) GAPDH acetylation in RAW264.7 cells treated with LPS/IFNγ for 16 h is abolished by the iNOS inhibitor 1400W. Cell lysates were immunoprecipitated with an anti-acetyl Lys antibody, and the immunoprecipitates were analysed by western blotting with an anti-GAPDH antibody. (b) Both p300 and CBP contribute to acetylation of GAPDH in U2OS cells treated with the NO donor, GSNO. Depletion of p300 or CBP by RNAi leads to diminished acetylation of GAPDH. (c) GAPDH mutation at Lys 160 abolishes its acetylation in the presence of 200 μM GSNO in HEK293 cells. (d) Acetylation of sulphonated GAPDH (sGAPDH) is observed only in the nucleus where it requires intact Lys 160. Cytosolic or nuclear fractions of HEK293 cells were immnoprecipiated with an anti-acetyl Lys antibody, and the immunoprecipitates were analysed by western blotting with a specific anti-sulphonated-GAPDH antibody. (e), R-(−)-Deprenyl (Dep) inhibits the acetylation of GAPDH in brains of mice treated with MPTP.