Abstract
Fasciclin family proteins have been identified as cell adhesion molecules in various organisms. In this study, a novel Magnaporthe oryzae fasciclin-like protein encoding gene, named MoFLP1, was isolated from a subtractive suppressive cDNA library and functionally analyzed. Sequence analysis showed that the MoFLP1 gene contains an open reading frame (ORF) of 1050 nucleotides encoding 349 amino acids with a calculated molecular weight of 35.85 kDa and a pI of 7.76. The deduced MoFLP1 protein contains a 17-amino acid secretion signal sequence and an 18-amino acid sequence with the characteristics of a glycosylphosphotidylinositol (GPI) anchor additional signal at its N- and C-terminuses, respectively. Potential N-glycosylation sites and domains involving cell adhesion were also identified in MoFLP1. Sequence analysis and subcellular localization by the expression of MoFLP1-GFP fusion construct in M. oryzae indicated that the MoFLP1 protein is probably localized on the vacuole membrane. Two MoFLP1 null mutants generated by targeted gene disruption exhibited marked reduction of conidiation, conidial adhesion, appressorium turgor, and pathogenicity. Our results indicate that fasciclin proteins play important roles in fungal development and pathogenicity in M. oryzae.
Keywords: Magnaporthe oryzae, Fasciclin, MoFLP1, Cellular localization, Conidiation, Pathogenicity
INTRODUCTION
The rice blast fungus, Magnaporthe oryzae, is responsible for significant losses in rice production worldwide and is considered as a model organism for studying fungus-plant interactions (Ou, 1985; Valent, 1990; Ebbole, 2007). The infection begins when the three-celled airborne conidia of M. oryzae is attached to the rice leaves. Germ tubes emerge from the conidia and subsequently differentiate into domeshaped cells called appressoria, which then directly penetrate the host cuticle using the enormous turgor pressure generated in them (de Jong et al., 1997). After penetration, the bulbous, branched infectious hyphae rapidly spread to adjacent cells and form conidiophores to release conidia into the environment to initiate new infection (Ou, 1985).
Fasciclin protein was first identified as a neuronal cell adhesion molecule in grasshopper (Bastiani et al., 1987). It is a glycosylphosphotidylinositol (GPI)-linked cell surface protein that mediates homophilic cell adhesion (Elkins et al., 1990). Up to now, multifarious fasciclin-like proteins have been found and identified in various species, including bacteria (Carr et al., 2003), algae (Huber and Sumper, 1994), lichens (Paulsrud and Lindblad, 2002), fungi (Miyazaki et al., 2007), animals (Kawamoto et al., 1998), and higher plants (Schultz et al., 2000; Johnson et al., 2003; Faik et al., 2006). Some vertebrate extracellular matrix (ECM) proteins from mammals, such as βig-h3 (Skonier et al., 1992), osteoblast-specific factor 2 (Takeshita et al., 1993), and RGD-CAP/βig-h3 (Hashimoto et al., 1997), were also identified as fasciclin-like proteins. In humans, stabilin-1 and stabilin-2, and FEEL-1 were also found to contain FAS1 domains (Politz et al., 2002; Adachi and Tsujimoto, 2002). Far-ranging existence of these fasciclin-like proteins implies that the proteins are evolutionarily conserved and have an important function. From various organisms, the fasciclin domain-containing proteins have been shown to function as adhesion molecules (Huber and Sumper, 1994; Kawamoto et al., 1998; Kim et al., 2000; Ohno et al., 2002; Sato et al., 2004). However, the biological function of the homologues of these proteins in phytopathogenic fungi is still unknown.
In the present study, we isolated a Magnaporthe fasciclin-like protein-encoding gene, namely, MoFLP1, and investigated its role in fungal development and pathogenicity in M. oryzae by targeted gene disruption. The deduced amino acid sequence of MoFLP1 contained homologous regions to the fasciclin domain of plant fasciclin-like arabinogalactan proteins (Johnson et al., 2003). Cellular localization indicates that MoFLP1-GFP transcripts were distributed distinctly in vacuoles, possibly located on the membrane vacuoles. Based on molecular characterization of the Δmoflp1 mutants, we found that a loss of MoFLP1 in M. oryzae led to significant reductions of conidiation, conidia adhension, and pathogenicity, which indicates that MoFLP1, a putative fasciclin-like protein, plays important roles in cell differentiation and pathogenicity in M. oryzae.
MATERIALS AND METHODS
Fungal strains and culture conditions
M. oryzae strain Guy11 and mutant strains were cultured on complete media (CM) (Talbot et al., 1993) at 25 °C with a 12-h photophase using fluorescent lights or cultivated in liquid medium at 25 °C in the dark with agitation (150~180 r/min). To investigate vegetative mycelial growth characteristics, the fungus was grown on three media: MM (minimal medium), MM-N (MM medium without the nitrogen source), and MM-C (MM medium without the carbon source) media (Liu et al., 2007).
DNA isolation and manipulation
Extraction of M. oryzae genomic DNA was carried out using a hexadecyltrimethylammonium bromide (CTAB) method as described by Talbot et al.(1993). Polymerase chain reaction (PCR), gel electrophoresis, restriction enzyme digestion and ligation reactions were all performed using standard procedures (Sambrook et al., 2002). Southern blot analysis was performed by using the digoxigenin (DIG) high prime DNA labeling and detection starter kit I (Roche, Germany).
Isolation and sequence analysis of MoFLP1
A cDNA clone (s98, GenBank accession No. CK828185) for MoFLP1 gene, with putative protein sequence homology to a fasciclin/βig-h3 protein, was isolated from a previously constructed Magnaporthe suppression subtractive cDNA library (Lu et al., 2005a). The cDNA fragment containing full open reading frame (ORF) of MoFLP1 was amplified from a mature Magnaporthe appressorium cDNA library (Lu et al., 2005b) using the forward primer 5′-CCTCCAGTCGCCCTTGTCACCCTCGTAA-3′ and the reverse primer 5′-GGCGCATCTTTCTTTTCCTTGGTCATCGTT-3′. The amplified product was cloned into a pGEM-T vector (Promega, USA), and sequenced.
The basic local alignment search tool (BLAST) algorithm in GenBank was used to search homology of nucleotide and amino acid sequences. Alignment of amino acid sequences was done using ClustalW (http://www.ebi.ac.uk/clustalw/) (Thompson et al., 1994). The signal peptide sequence and cleavable site of MoFLP1 were estimated by SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/) (Dyrløv Bendtsen et al., 2004). N-glycosylation sites of MoFLP1 were identified using PROSITE (http://www.au.expancy.org/prosite) (Bairoch et al., 1997). The C-terminal GPI anchor and cleavage site were predicted using big-PI predictor (http://www.mendel.imp.ac.at/gpi/gpi_server.html) (Eisenhaber et al., 1998).
Generation of the MoFLP1-GFP fusion construct under control of the NAR promoter
A 1.4-kb NotI-SpeI hygromycin resistance gene (HPH) fragment was inserted into the NotI-SpeI site of pEGFP vector (Clontech, USA) to produce plasmid pEGFP-HPH. The HPH gene was cloned from plasmid pCB1003 (Carroll et al., 1994) using primers 5′-CCgcggccgcCTACTCTATTCCTTTGCCCTCG-3′ and 5′-CCactagtTGGAGGTCAACACATCAAT-3′. The promoter fragment of the NAR gene was cloned from the plasmid pNAR (Lu et al., 2007a) by PCR using primers 5′-CAtctagaGGGAAGCGATTGCGTT-3′ and 5′-GGggatccCCATGGTGTCGGTTGTGGTG-3′ and inserted into the XbaI-BamHI site of pEGFP-HPH to produce pNAR-EGFP vector. Subsequently, the coding domain of the MoFLP1 gene without the stop codon (TAA) was amplified with primers 5′-TTGGTACCATGAAATTTACTATTACCGTCT-3′ and 5′-TTACCGGTAGACCCGTAATAATAGCGACGC-3′ and inserted into the KpnI-AgeI site of pNAR-EGFP to generate pMoFLP1-GFP. The resulting vector, namely, pMoFLP1-GFP, was introduced into protoplasts of Guy11 after linearization by XbaI. Single-copy genomic integration of the resulting transformants was confirmed by Southern blot analysis. Fluorescence signal of the transformants was detected using a Leica TCS SP5 inverted confocal laser scanning microscope (Leica, Germany).
Construction of MoFLP1 gene disruption vector and fungal transformation
To analyze the function of MoFLP1 gene in M. oryzae, a targeted gene disruption vector pBS-MoFLP1 was constructed by inserting two flanking sequences of MoFLP1 gene into the pBS-HPH1 vector (Liu et al., 2007). First, a 1.3-kb KpnI-XhoI fragment from the 5′ end of the MoFLP1 gene obtained by PCR with primers s98upp1 (5′-AAggtaccTCATTGAGGTTACCGGGGATTAGG-3′) and s98upp2 (5′-AAGGGCAGAGTTTACACAGGAAGG-3′) was inserted into the KpnI-XhoI site of pBS-HPH1 to produce plasmid pMoFLP1-UP-HPH. Then a 1.5-kb XbaI-NotI fragment from the 3′ end of the MoFLP1 gene was amplified with primers s98downp1 (5′-AAtctagaTGATGGTCGGCGTCGCTATTATTAC-3′) and s98downp2 (5′-AAgcggccgcGGGGGCTGGCAAGGTGTCG-3′) and inserted into the XbaI-NotI site of pMoFLP1-UP-HPH to produce pBS-MoFLP1. After linearization by HindIII, the targeted gene disruption vector, namely, pBS-MoFLP1, was introduced into the protoplasts of Guy11. Protoplast preparation and transformation were performed by previously established procedures with some modification (Talbot et al., 1993; Lu et al., 2007b). After incubation for 7 d in the dark, transformants were transferred to CM plates with hygromycin B for further selection.
Identification of MoFLP1 null mutants
Gene deletion mutants were identified initially by PCR using the checking primers s98checkp1 (5′-GGGGAGCGCCAAGAACATCACCATC-3′) and s98checkp2 (5′-AGCGGCGGGCGGAAGAGTCAAG-3′), which are internal to MoFLP1. The putative gene deletion mutants were purified by single-spore isolation and the single integration event was confirmed by Southern blot analysis.
Vegetative growth, conidiation, appressorium formation, and conidial adhesion assays
Assays for vegetative growth, conidiation, appressorium formation, and appressorium turgor were performed by established protocols as described previously (Lu et al., 2007b; Liu TB et al., 2008). Conidial development analysis was performed as described by Yi et al.(2008) and conidial adhesion was analyzed according to the method proposed by Ahn et al.(2004).
Pathogenicity tests
Pathogenicity tests were also carried out by the established protocols as previously described (Lu et al., 2007b). Two-week-old rice seedlings (cv CO-39) and eight-day-old barley leaves (cv ZJ-8) were inoculated by spraying conidial suspension (1×105 conidia/ml) and then placed in a growth chamber with a 12-h photophase using fluorescent lights for 7 d. Disease severity was recorded according to the method proposed by Bonman et al.(1986).
RESULTS
Isolation of MoFLP1
An expressed sequence tag (EST) (s98) encoding an M. oryzae fasciclin-like protein (MoFLP1) was found in a Magnaporthe subtractive suppressive cDNA library (Lu et al., 2005a). After searching the M. oryzae database (http://www.broad.mit.edu/annotation/fungi/magnaporthe/) using BLAST algorithm, this EST was found in corresponding to a hypothetical protein, MGG_02884.6, with a 100% nucleotide identity in strain 70-15.
To find out the potential role of MoFLP1, we isolated a full-length MoFLP1 gene from the mature appressorium cDNA library (Lu et al., 2005b) by PCR. Sequence analysis (GenBank accession No. FJ608586) revealed that MoFLP1 is 1050 bp long, encoding a 349-amino acid protein with a calculated molecular weight of 35.85 kDa and a pI of 7.76. As an expected transmembrane protein, the predicted MoFLP1 protein contains both a putative signal peptide protein consisting of 17 mainly hydrophobic amino acids in the N-terminus (Fig.1) (Dyrløv Bendtsen et al., 2004) and an 18-amino acid potential signal sequence for a GPI anchor in the C-terminus (Fig.1) (Eisenhaber et al., 1998). Four potential N-glycosylation sites were also identified in MoFLP1 (Fig.1) (Bairoch et al., 1997). A homology search of the database suggested that MoFLP1 contains a characteristic region, homologous to the fasciclin domain found in fungal and plant fasciclin-like proteins (Miyazaki et al., 2007; Johnson et al., 2003; Dahiya et al., 2006; Liu D et al., 2008). H1 and H2, the important regions involved in cell adhesion in the fasciclin domain (Kawamoto et al., 1998), and the putative adhesion motif located between the H1 and H2 regions (Liu D et al., 2008) were also identified within the fasciclin-like domain of MoFLP1 (Fig.1).
Fig. 1.
Alignment of the predicted MoFLP1 protein sequence with fungal homologues
The sequences are MoFLP1 of M. oryzae (Mo, GenBank accession No. FJ608586), homologues of Pyrenophora tritici-repentis (Pt, GenBank accession No. XP_001942181), Phaeosphaeria nodorum (Pn, GenBank accession No. XP_001798270), and Fusarium graminearum (Fg, GenBank accession No. XP_382301). Sequences were aligned with ClustalW program and manually edited. The number of amino acids of each protein is given at the right of the alignment. Identical residues in all sequences are indicated by asterisks. Dashes indicate the gaps in all sequences. Dots and colons indicate semiconserved and conservative substitutions in all sequences, respectively. The conserved regions characteristic of fasciclin domains, H1 and H2, are indicated below the alignment and amino acids that are thought to be involved in adhesion are boxed and indicated above the alignment. The sequence in the N-terminus indicating a predicted signal peptide is underlined. The conserved potential N-glycosylation sites (NIT, NGT, NAT, and NGT) are underlined by black circles and the attachment/cleavage sites in the putative GPI anchor additional sequence are indicated by black triangles
As of this writing, no analysis of a fungal fasciclin-like protein had been reported in ascomycetous fungi, whereas several amino acid sequences of presumable homologues of MoFLP1 were found in databases. An amino acid sequence alignment of MoFLP1 with three putative plant pathogenic fungi homologues obtained by the maximum parsimony analysis is shown in Fig.1. These proteins are homologous throughout, and H1 and H2 were partially conserved in comparison to a consensus sequence generated from 78 fasciclin proteins in various species (Kawamoto et al., 1998).
Cellular localization of MoFLP1
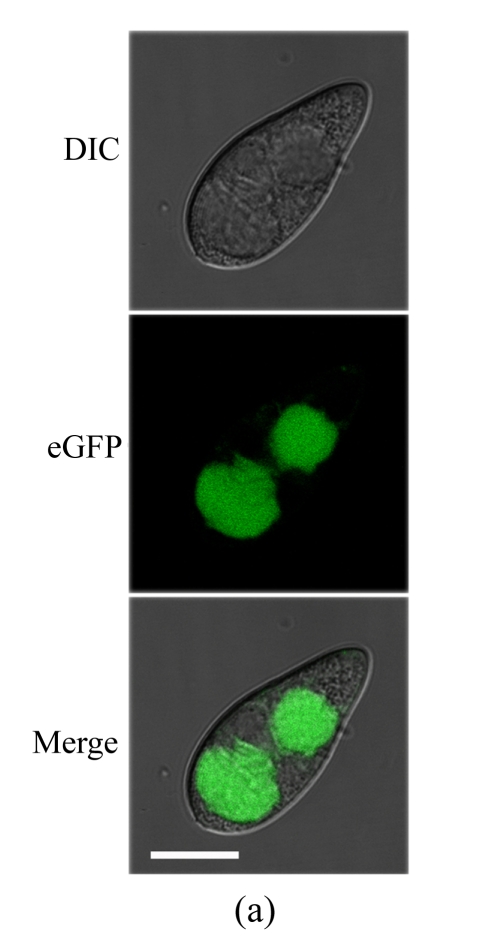
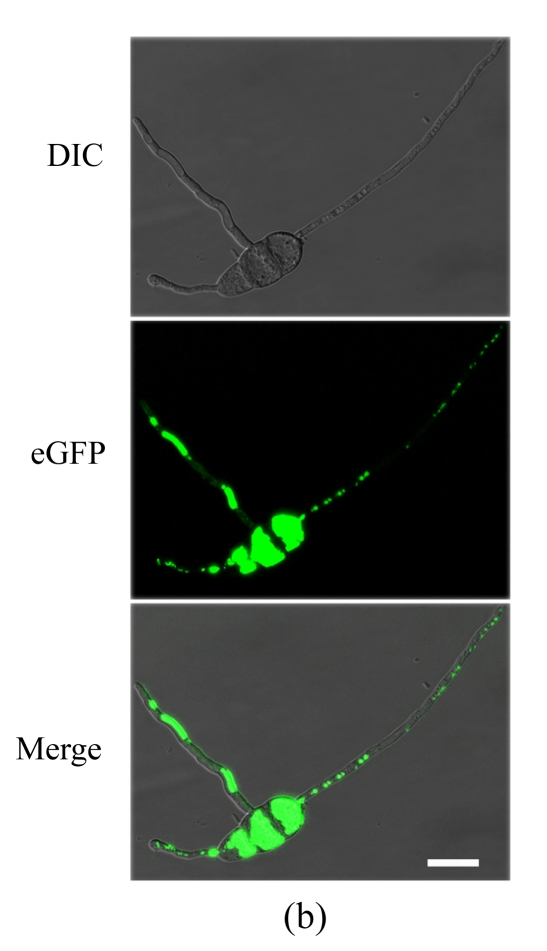
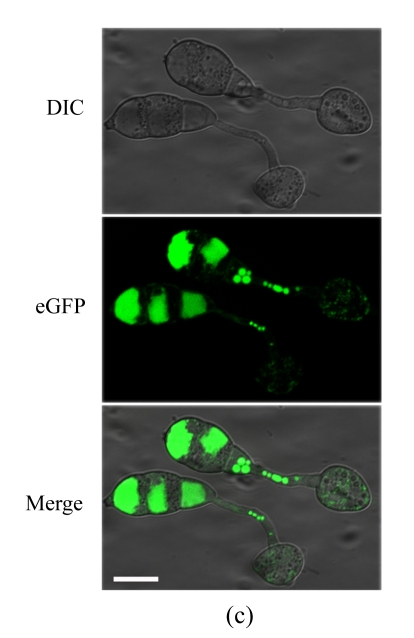
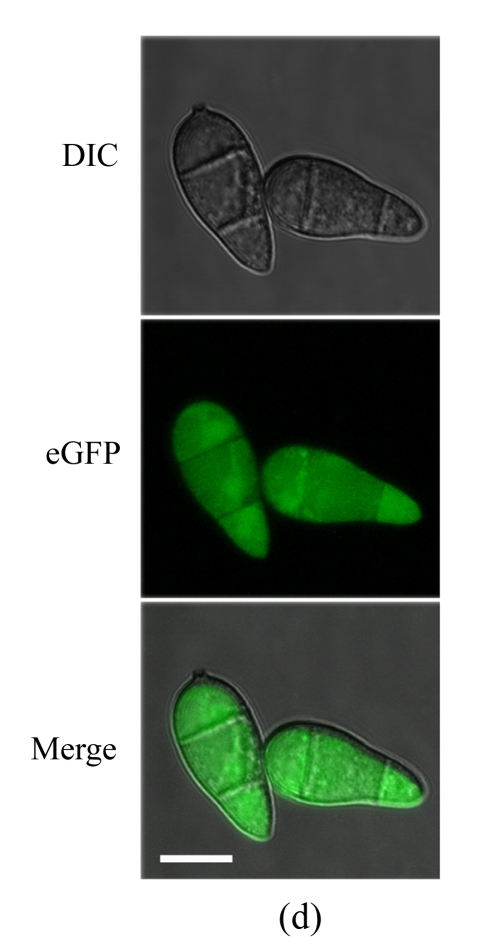
To determine the cellular localization of MoFLP1 in M. oryzae, we constructed a MoFLP1-fluorescent green protein gene fusion construct (pMoFLP1-GFP) under control of the NAR promoter (Lu et al., 2007a). The MoFLP1-GFP fusion construct linearized with XbaI was transformed into the protoplasts of Guy11 strain. Two strains carrying a single copy integration of pMoFLP1-GFP were confirmed by Southern blot analysis (data not shown). GFP detection was performed by using confocal laser scanning fluorescence microscopy. GFP fluorescence observation showed that the MoFLP1-GFP fusion protein was located in the vacuoles of conidia, mycelia, and appressoria of the transformant NMG2 (Fig.2 in p.440). When pGFP-ATG1 (Liu et al., 2007), a control green fluorescent protein fusion expression vector built with the same strategy as pMoFLP1-GFP, was transformed into the Guy11 strain, the fluorescence was typically localized in the cytoplasm (Fig.2d). Based on the sequence analysis results and the localization pattern described above, we predicted that the MoFLP1 protein possibly was localized on the membrane of the vacuoles.
Fig. 2.
Cellular localization of MoFLP1 in M. oryzae. MoFLP1-GFP protein was detected in the vacuoles of M. oryzae strain NMG2
The conidia (a), germinated conidia (b) at 10 h post-incubation, and appressoria (c) at 6 h post-incubation of strain NMG2 were observed by inverted confocal laser scanning microscope. Representative bright-field [differential interference contrast (DIC), top], fluorescence (middle), and merged images (bottom) are shown. (d) The conidia of the Guy11 strain transformed with pGFP-ATG1 were observed by inverted confocal laser scanning microscope and ATG1-GFP appeared in the cytoplasm. Bar=10 μm
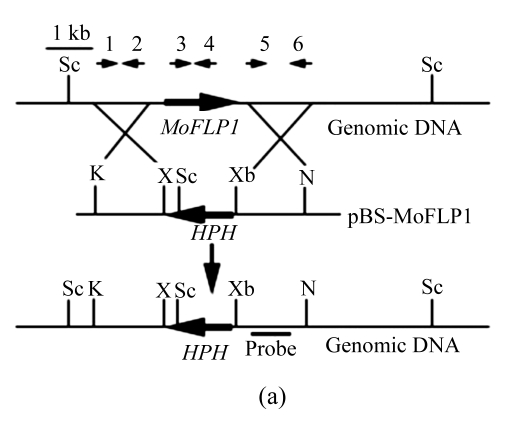
Disruption of MoFLP1
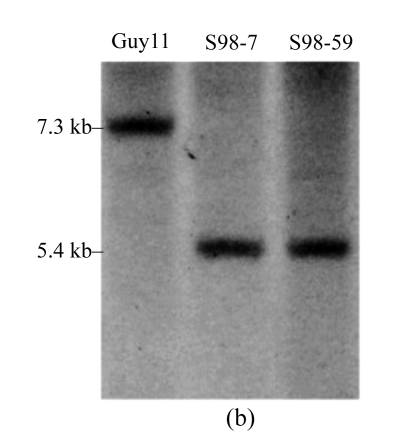
To determine the role of MoFLP1 at the molecular level, a targeted gene disruption experiment was adopted to obtain MoFLP1 null mutants. The gene disruption vector, pBS-MoFLP1 (Fig.3a), was transformed into the protoplasts of Guy11 strain after confirmation by sequencing and linearization by HindIII. Eighty-nine hygromycin-resistant transformants were obtained and screened by PCR using primers, s98checkp1 and s98checkp2, internal to MoFLP1. Two of these transformants were confirmed to have lost their MoFLP1 genes and subsequently purified by single-spore isolation. In Southern blot analysis, a 5.4-kb fragment was detected in the two transformants in contrast with a 7.3-kb fragment in the wild-type strain. The band shift from 7.3 to 5.4 kb indicated that MoFLP1 had been replaced by HPH gene in mutants s98-7 and s98-59 (Fig.3b). The mutants were further confirmed by the presence of HPH gene, as defined by PCR with HPH specific primers (data not shown).
Fig. 3.
Targeted disruption of the MoFLP1 gene in M. oryzae
(a) MoFLP1 locus and disruption vector (pBS-MoFLP1). The orientations of the MoFLP1 and HPH genes are indicated by large arrows. The orientations and positions of primers s98upp1, s98upp2, s98checkp1, s98checkp2, s98downp1, and s98downp2 are indicated as 1, 2, 3, 4, 5, and 6, respectively, with small arrows. The gene disruption construct was digested with HindIII and introduced into the protoplasts of M. oryzae Guy11. K=KpnI, N=NotI, X=XhoI, Sc=ScaI, Xb=XbaI; (b) Southern blot analysis of the MoFLP1 disrupted transformants. All genomic DNAs were digested with ScaI, fractionated and hybridized with a 623-bp fragment located in the downstream flanking sequence of MoFLP1 shown in Fig.2a. As expected, a 5.4-kb band was detected in Δmoflp1 mutants s98-7 and s98-59 in contrast with a 7.3-kb band in the wild-type strain Guy11
Effect of MoFLP1 on conidial development and appressorial turgor pressure in M. oryzae
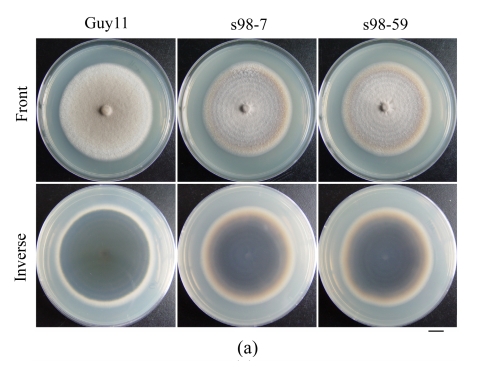
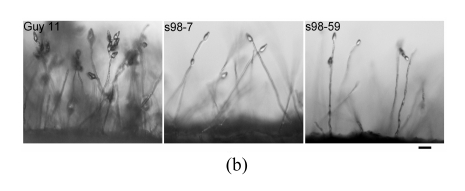
The growth rates of the Δmoflp1 null mutants (s98-7 and s98-59) were indistinguishable (P≤0.01) from the wild-type strain Guy11 when cultured on CM plates. However, in contrast to the dense aerial hyphae in the wild-type strain, the Δmoflp1 null mutants produced sparse aerial hyphae on CM plates (Fig.4a). Meanwhile, conidiation in Δmoflp1 mutants was reduced significantly. The two independently generated mutants produced fewer conidia, approximately 8% of those in the wild-type (Table 1). To observe the conidial development, agar slices containing mycelia were cut and transferred to 2% water agar plates after scraping away the aerial hyphae and kept under constant illumination at 25 °C. At 24 h post-incubation, the wild-type strain developed dense aerial hyphae with 3~5 conidia, while both of the Δmoflp1 mutants produced fewer aerial hyphae and single developed conidia (Fig.4b). In addition, we also observed a significant reduction (~15%) in conidial adhesion to the hydrophobic surface of GelBond (Table 1). However, targeted disruption of MoFLP1 had no effect on conidial germination and appressorium formation (Table 1). Interestingly, appressorium turgor pressure was affected by disruption of MoFLP1. When exposed to 2 mol/L glycerol solution 24 h after incubation, approximately half of the appressoria in the two Δmoflp1 mutants had collapsed in comparison to 23.21% of the appressoria in wild-type Guy11 (Table 1). The growth of the Δmoflp1 mutants was also assessed on MM-C and MM-N media and was comparable (P≤0.05). These results indicate that the Δmoflp1 mutants are not sensitive to such starvation and the MoFLP1 gene is not necessary for normal growth under such circumstances.
Fig. 4.
Colonial morphology and conidial development of M. oryzae strains
(a) The wild-type Guy11 and Δmoflp1 mutants s98-7 and s98-59 were cultured on CM plates for 10 d and photographed. Bar=1 cm; (b) Conidial development assay was performed as described by Yi et al.(2008) and conidiation was observed under a light microscope after 24 h. Bar=30 μm
Table 1.
Characteristics of Δmoflp1 mutants in fungal development
| Conidiation (104 cm−2)a | Conidial adhesion (%)b | Conidial germination (%)c |
Appressorium formation (%)d |
Appresorium turgor (%)e | ||||
| 2 h | 4 h | 6 h | 12 h | 24 h | ||||
| Guy11 | 91.33±7.12A | 85.65±4.72A | 88.76±1.80A | 98.33±0.38A | 79.34±3.25A | 96.60±1.84A | 98.91±1.22A | 23.21±2.23A |
| Mutants | ||||||||
| S98-7 | 7.91±0.75B | 70.43±5.66B | 90.34±2.95A | 98.96±0.47A | 79.97±2.26A | 95.40±0.95A | 98.68±0.89A | 45.05±1.69B |
| S98-59 | 7.82±0.34B | 71.67±7.25B | 89.46±2.86A | 99.08±0.80A | 80.25±1.38A | 96.15±1.71A | 97.43±2.38A | 47.35±2.48B |
Mycelial plugs measuring 1 cm diameter were taken from 10-d-old colonies cultured on CM plates. Conidia were collected in 1 ml of distilled water and counted with a haemacytometer under a microscopy;
10-d-old conidia of each strain were harvested and conidial concentrations were adjusted to 5×105 conidia/ml with distilled water. 5 μl of suspension was placed on GelBond film and incubated in a humid box. Conidial adhesion was measured after 2 h as described by Ahn et al.(2004);
10-d-old conidia of each strain were harvested and conidial concentrations were adjusted to 1×105 conidia/ml with distilled water. Conidial germination was measured after 2 and 4 h;
10-d-old conidia of each strain were harvested and conidial concentrations were adjusted to 1×105 conidia/ml with distilled water. Appressorium formation was measured after 6, 12, and 24 h on duplicate films as described previously (Liu TB et al., 2008);
Appressorium turgor was measured as described previously (Lu et al., 2007). The proportion of collapsed appressoria was recorded after exposure to 2 mol/L glycerol solution for 10 min;
The same superscript capital letters in each column are not significantly different, as estimated by the Duncan’s test (P≤0.01)
Effect of MoFLP1 on full virulence
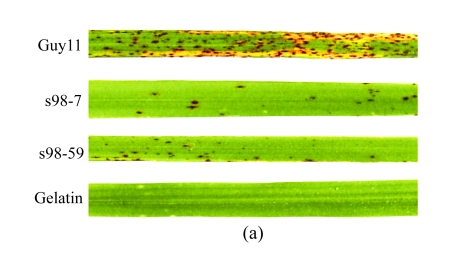
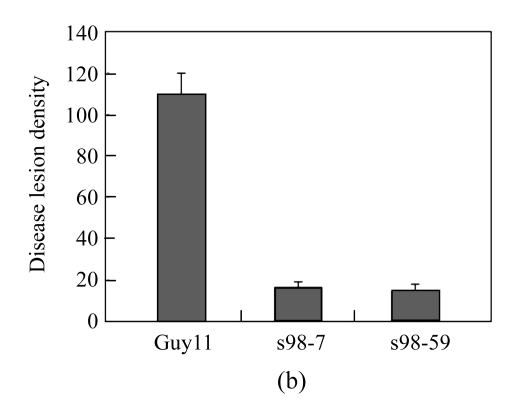
To evaluate the pathogenicity of Δmoflp1 mutants to the susceptible host, rice plants were inoculated by spraying with the conidia of wild-type and two Δmoflp1 mutants. The lesions caused by the wild-type strain are typical spindle-like and gray-centered, most of which coalesced later, whereas those caused by the Δmoflp1 mutants were small and isolated (Fig.5a). Severity of disease caused by Δmoflp1 mutants reduced significantly. Plants inoculated with the Δmoflp1 mutants were graded as 1 to 2, with more than 90% of the plants scored as 1, in contrast to 90% of those inoculated with the wild-type scored as 4 to 5. In a 5-cm section of leaf, the disease lesion density caused by the Δmoflp1 mutants was only ~15% of that caused by the wild-type strain (Fig.5b, P≤0.01). Similar results were obtained when spraying on barley leaves with the conidia of the wild-type and two Δmoflp1 mutants (data not shown). Consequently, MoFLP1 was vital for full virulence in M. oryzae.
Fig. 5.
Pathogenicity of Δmoflp1 mutants on rice
(a) Seedlings of rice were inoculated with wild-type Guy11 strain, Δmoflp1 mutants (S98-7 and S98-59), and gelatin (0.2% (w/v), control). Disease symptoms were observed 7 d after inoculation; (b) Disease lesion density of inoculated rice seedlings in a 5-cm section of leaf. Values are expressed as mean±SD of three experiments
DISCUSSION
In this study, we analyzed a novel fungal fasciclin-like protein-encoding gene, MoFLP1, isolated from M. oryzae using a PCR-based strategy and investigated the contribution of fasciclin-like protein in conidiation and pathogenicity of M. oryzae using targeted gene disruption strategy. The experimental results suggest that MoFLP1 has a characteristic region homologous to the fasciclin domain found in fasciclin family proteins and that the MoFLP1 gene is required for the conidiation and pathogenicity of M. oryzae.
Fasciclin family proteins have already been found in a variety of organisms (Huber and Sumper, 1994; Paulsrud and Lindblad, 2002; Carr et al., 2003; Johnson et al., 2003), in which they promote cell-to-cell adhesion (Huber and Sumper, 1994; Kawamoto et al., 1998; Kim et al., 2000; Sato et al., 2004). Recently, a fungal fasciclin-like protein-encoding gene Le.flp1 was isolated from the basidiomycetous mushroom Lentinus edodes and functionally analyzed, which suggests that Le.flp1 plays a role in cell differentiation and development in ubiquitous tissues during fruiting body formation possibly through cell adhesion (Miyazaki et al., 2007). Besides the putative fungal fasciclin proteins (Fig.1), there are several putative ascomycetous homologues of MoFLP1 in databases. However, no analysis of fungal fasciclin family proteins has been reported in ascomycetes yet.
In the plant Zinnia elegans, ZeFLA11, a fasciclin domain-containing protein, has been predicted to have both an N-terminal signal peptide and a cleavable signal peptide with a C-terminus site for the GPI anchor, and to function as a cell-surface GPI-anchored glycoprotein (Dahiya et al., 2006). In the present study, the MoFLP1 and other fungal homologues (Fig.1) seem to contain both a hydrophobic signal peptide with a potential cleavable site in the N-terminus (Dyrløv Bendtsen et al., 2004) and a GPI anchor site in the C-terminus (Eisenhaber et al., 1998). These results suggest that MoFLP1 may work as an extracellular and/or membrane-bound protein. MoFLP1-GFP fusion expression experiments showed that the MoFLP1 protein was located at the vacuoles. Considering the computer program-predicted results and subcellular localization analysis, we speculated that the MoFLP1 protein might be located on the membrane of the vacuoles.
In M. oryzae, targeted deletion of fasciclin-like protein-encoding gene, MoFLP1, resulted in markedly reduced production of conidia. The conidial formation in the rice blast fungus is a complicated process, affected by many known signal pathways, such as cyclic adenosine monophosphate (cAMP) signaling (Adachi and Hamer, 1998), mitogen-activated protein (MAP) kinase (Zhao et al., 2007), and autophagy pathways (Veneault-Fourrey et al., 2006; Liu et al., 2007). The conidiation was also affected by deletion of the following genes such as funga1 hydrophobin gene MHP1 (Kim et al., 2005), PAK kinase genes CHM1 and MST20 (Li et al., 2004), G-beta subunit gene MGB1 (Nishimura et al., 2003), a blue light receptor gene MgWC-1 (Lee et al., 2006), and a type III integral transmembrane protein encoding gene MTP1 (Lu et al., 2008). Although the mechanism of conidiation reduction in Δmoflp1 mutants remains unclear, it was reported that conidium formation relies on the endogenous sources for nutrient supply through autophagy in Aspergillus oryzae or M. oryzae (Kikuma et al., 2006; Liu et al., 2007). Therefore, further study is still needed to confirm the relationships between MoFLP1 gene and other known signal pathways such as autophagy.
Targeted disruption of MoFLP1 also resulted in remarkably reduced pathogenicity on rice. There are two possible explanations for this. First, deletion of MoFLP1 gene resulted in a significant reduction of conidial adhesion to the hydrophobic surface of GelBond. In animals, it has been suggested that fasciclin/βig-h3 may act as a cell adhesion substrate (Park et al., 2004) and promote cell attachment activity (Sato et al., 2004). In plants, fasciclin-like arabinogalactan proteins have been implicated to be associated with plant growth and development in various processes, including embryogenesis, cell adhesion, and cell proliferation (Schultz et al., 2000; Johnson et al., 2003). In M. oryzae, deletion of an extracellular matrix protein encoding gene, EMP1, also resulted in the reduction of conidial adhesion and pathogenicity (Ahn et al., 2004). Appressorium penetration may require stronger adhesion to break the cuticle layer and the epidermal cell wall. Thus, the reduction of conidial adhesion may result in the reduction of pathogenicity on rice. Second, deletion of MoFLP1 resulted in a reduction of appressorium turgor in Δmoflp1 mutants, which may cause the decreased penetration ability. Disruption of nonhistone protein coding gene MNH6 and autophagy-related gene MgATG1 in M. oryzae also caused a reduction of appressorium turgor, leading to the loss of the ability to penetrate plants (Lu et al., 2007b; Liu et al., 2007).
In conclusion, the MoFLP1 gene encodes a fasciclin-like protein and plays significant roles in conidiation, conidial adhesion, and pathogenicity in M. oryzae. Identification of MoFLP1 as a novel fasciclin-like protein, which was involved in above fungal developmental stages, may be very helpful to find fungal signaling pathways and new strategies to control rice blast disease. Future studies are needed to further examine the roles of MoFLP1 in other signaling pathways, such as autophagy, in conidiation and pathogenicity of M. oryzae.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30870101) and the Public Welfare Profession (Agriculture) Research Project (No. 200803008), China
References
- 1.Adachi H, Tsujimoto M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J Biol Chem. 2002;277(37):34264–34270. doi: 10.1074/jbc.M204277200. [DOI] [PubMed] [Google Scholar]
- 2.Adachi K, Hamer JE. Divergent camp signaling pathways regulate growth and pathogenesis in the rice blast fungus, Magnaporthe grisea . Plant Cell. 1998;10(8):1361–1373. doi: 10.1105/tpc.10.8.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn N, Kim S, Choi W, Im KH, Lee YH. Extracellular matrix protein gene, EMP1, is required for appressorium formation and pathogenicity of the rice blast fungus, Magnaporthe grisea . Mol Cells. 2004;17(1):166–173. [PubMed] [Google Scholar]
- 4.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997;25(1):217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastiani MJ, Harrelson AL, Snow PM, Goodman CS. Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell. 1987;48(5):745–755. doi: 10.1016/0092-8674(87)90072-9. [DOI] [PubMed] [Google Scholar]
- 6.Bonman JM, Vergel DDT, Khin MM. Physiologic specialization of Pyricularia oryzae in the Philippines. Plant Dis. 1986;70(8):767–769. doi: 10.1094/PD-70-767. [DOI] [Google Scholar]
- 7.Carr MD, Bloemink MJ, Dentten E, Whelan AO, Gordon SV, Kelly G, Frenkiel TA, Hewinson RG, Williamson RA. Solution structure of the Mycobacterium tuberculosis complex protein MPB70: from tuberculosis pathogenesis to inherited human corneal desease. J Biol Chem. 2003;278(44):43736–43743. doi: 10.1074/jbc.M307235200. [DOI] [PubMed] [Google Scholar]
- 8.Carroll AM, Sweigard JA, Valent B. Improved vectors for selecting resistance to hygromycin. Fungal Genet Newslett. 1994;41:22. [Google Scholar]
- 9.Dahiya P, Findlay K, Roberts K, McCann MC. A fasciclin-domain containing gene, ZeFLA11, is expressed exclusively in xylem elements that have reticulate wall thickenings in the stem vascular system of Zinnia elegans cv Envy. Planta. 2006;223(6):1281–1291. doi: 10.1007/s00425-005-0177-9. [DOI] [PubMed] [Google Scholar]
- 10.de Jong JC, McCormack BJ, Smirnoff N, Talbot NJ. Glycerol generates turgor in rice blast. Nature. 1997;389(6648):244–245. doi: 10.1038/38418. [DOI] [Google Scholar]
- 11.Dyrløv Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340(4):783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Ebbole DJ. Magnaporthe as a model for understanding host-pathogen interactions. Annu Rev Phytopathol. 2007;45(1):437–456. doi: 10.1146/annurev.phyto.45.062806.094346. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhaber B, Bork P, Eisenhaber F. Sequence properties of GPI-anchored proteins near the omega-site: constraints for the polypeptide binding site of the putative transamidase. Protein Engineering Design and Selection. 1998;11(12):1155–1161. doi: 10.1093/protein/11.12.1155. [DOI] [PubMed] [Google Scholar]
- 14.Elkins T, Hortsch M, Bieber AJ, Snow PM, Goodman CS. Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. J Cell Biol. 1990;110(5):1825–1832. doi: 10.1083/jcb.110.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faik A, Abouzouhair J, Sarhan F. Putative fasciclin-like arabinogalactan-proteins (FLA) in wheat (Triticum aestivum) and rice (Oryza sativa): identification and bioinformatic analyses. Mol Gen Genomics. 2006;276(5):478–494. doi: 10.1007/s00438-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Noshiro M, Ohno S, Kawamoto T, Satakeda H, Akagawa Y, Nakashima K, Okimura A, Ishida H, Okamoto T, et al. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/beta ig-h3) that binds to collagen. Biochim Biophys Acta. 1997;1355(3):303–314. doi: 10.1016/S0167-4889(96)00147-4. [DOI] [PubMed] [Google Scholar]
- 17.Huber O, Sumper M. Algal-CAMs: isoforms of a cell adhesion molecule in embryos of the alga volvox with homology to Drosophila fasciclin I. EMBO J. 1994;13(18):4212–4222. doi: 10.1002/j.1460-2075.1994.tb06741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KL, Jones BJ, Bacic A, Schultz CJ. The fasciclin-like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiol. 2003;133(4):1911–1925. doi: 10.1104/pp.103.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamoto T, Noshiro M, Shen M, Nakamasu K, Hashimoto K, Kawashima-Ohya Y, Gotoh O, Kato Y. Structural and phylogenetic analyses of RGD-CAPrbig-h3, a fasciclin-like adhesion protein expressed in chick chondrocytes. Biochimica et Biophysica Acta. 1998;1395:288–292. doi: 10.1016/S0167-4781(97)00172-3. [DOI] [PubMed] [Google Scholar]
- 20.Kikuma T, Ohneda M, Arioka M, Kitamoto K. Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae . Eukaryot Cell. 2006;5(8):1328–1336. doi: 10.1128/EC.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, beta-ig-h3. J Biol Chem. 2000;275(40):30907–30915. doi: 10.1074/jbc.M002752200. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Ahn IP, Rho HS, Lee YH. MHP1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol Microbiol. 2005;57(5):1224–1237. doi: 10.1111/j.1365-2958.2005.04750.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee K, Singh P, Chung WC, Ash J, Kim TS, Hang L, Park S. Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae . Fungal Genet Biol. 2006;43(10):694–706. doi: 10.1016/j.fgb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Xue C, Bruno K, Nishimura M, Xu JR. Two PAK kinase genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea . Mol Plant Microbe Interact. 2004;17(5):547–556. doi: 10.1094/MPMI.2004.17.5.547. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Tu L, Li Y, Wang L, Zhu L, Zhang X. Genes encoding fasciclin-like arabinogalactan proteins are specifically expressed during cotton fiber development. Plant Mol Biol Rep. 2008;26(2):98–113. doi: 10.1007/s11105-008-0026-7. [DOI] [Google Scholar]
- 26.Liu TB, Lu JP, Min H, Lin FC. A simple and effective method for total RNA isolation of appressoria in Magnaporthe oryzae . J Zhejiang Univ Sci B. 2008;9(10):811–817. doi: 10.1631/jzus.B0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XH, Lu JP, Zhang L, Dong B, Min H, Lin FC. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryot Cell. 2007;6(6):997–1005. doi: 10.1128/EC.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu JP, Liu TB, Lin FC. Identification of mature appressorium-enriched transcripts in Magnaporthe grisea, the rice blast fungus, using suppression subtractive hybridization. FEMS Microbiol Lett. 2005;245(1):131–137. doi: 10.1016/j.femsle.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Lu JP, Liu TB, Liu XY, Lin FC. Representative appressorium stage cDNA library of Magnaporthe grisea . J Zhejiang Univ Sci B. 2005;6(2):132–136. doi: 10.1631/jzus.2005.B0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu JP, Duan ZB, Liu TB, Lin FC. Cloning, sequencing and expression analysis of the NAR promoter activated during hyphal stage of Magnaporthe grisea . J Zhejiang Univ Sci B. 2007;8(9):661–665. doi: 10.1631/jzus.2007.B0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu JP, Feng XX, Liu XH, Lu Q, Wang HK, Lin FC. Mnh6, a nonhistone protein, is required for fungal development and pathogenicity of Magnaporthe grisea . Fungal Genet Biol. 2007;44(9):819–829. doi: 10.1016/j.fgb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Lu Q, Lu JP, Li XD, Liu XH, Min H, Lin FC. Magnaporthe oryzae MTP1 gene encodes a type III transmembrane protein involved in conidiation and conidial germination. J Zhejiang Univ Sci B. 2008;9(7):511–519. doi: 10.1631/jzus.B0820015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazaki Y, Kaneko S, Sunagawa M, Shishido K, Yamazaki T, Nakamura M, Babasaki K. The fruiting-specific Le.flp1 gene, encoding a novel fungal fasciclin-like protein, of the basidiomycetous mushroom Lentinula edodes . Curr Genet. 2007;51(6):367–375. doi: 10.1007/s00294-007-0133-2. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura M, Park G, Xu JR. The G-beta subunit MGB1 is involved in regulating multiple steps of infection-related morphogenesis in Magnaporthe grisea . Mol Microbiol. 2003;50(1):231–243. doi: 10.1046/j.1365-2958.2003.03676.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohno S, Doi T, Tsutsumi S, Okada Y, Yoneno K, Kato Y, Tanne K. RGD-CAP (βig-h3) is expressed in precartilage condensation and in prehypertrophic chondrocytes during cartilage development. Biochim Biophys Acta. 2002;1572(1):114–122. doi: 10.1016/s0304-4165(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 36.Ou SH. Rice Diseases. 2nd Ed. Kew, UK: Commonwealth Mycological Institute; 1985. [Google Scholar]
- 37.Park SW, Bae JS, Kim KS, Park SH, Lee BH, Choi JY, Park JY, Ha SW, Kim YL, Kwon TH, et al. Beta ig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with α3β1 integrin. Exp Mol Med. 2004;36(3):211–219. doi: 10.1038/emm.2004.29. [DOI] [PubMed] [Google Scholar]
- 38.Paulsrud P, Lindblad P. Fasciclin domain proteins are present in nostoc symbionts of lichens. Appl Environ Microbiol. 2002;68(4):2036–2039. doi: 10.1128/AEM.68.4.2036-2039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Politz O, Gratchev A, McCourt PAG, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowaka J, et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362(1):155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 3rd Ed. New York, N.Y: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 41.Sato K, Nishi N, Nomizu M. Characterization of a fasciclin I-like protein with cell attachment activity from sea urchin (Strongylocentrotus intermedius) ovaries. Arch Biochem Biophys. 2004;424(1):1–10. doi: 10.1016/j.abb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Schultz CJ, Johnson KL, Currie G, Bacic A. The classical arabinogalactan protein gene family of Arabidopsis . Plant Cell. 2000;12(9):1751–1767. doi: 10.1105/tpc.12.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992;11(7):511–522. doi: 10.1089/dna.1992.11.511. [DOI] [PubMed] [Google Scholar]
- 44.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294(1):271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talbot NJ, Ebbole DJ, Hamer JE. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea . Plant Cell. 1993;5(11):1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valent B. Rice blast as a model system for plant pathology. Phytopathology. 1990;80(1):33–36. doi: 10.1094/Phyto-80-33. [DOI] [Google Scholar]
- 48.Veneault-Fourrey C, Barooah MK, Egan MJ, Talbot NJ. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science. 2006;312(5773):580–583. doi: 10.1126/science.1124550. [DOI] [PubMed] [Google Scholar]
- 49.Yi M, Park JH, Ahn JH, Lee YH. MoSNF1 regulates sporulation and pathogenicity in the rice blast fungus Magnaporthe oryzae . Fungal Genet Biol. 2008;45(8):1172–1181. doi: 10.1016/j.fgb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Zhao X, Mehrabi R, Xu JR. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell. 2007;6(10):1701–1714. doi: 10.1128/EC.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]